Yap/Taz Deletion in Gli+ Cell-Derived Myofibroblasts Attenuates Fibrosis - PubMed (original) (raw)
Yap/Taz Deletion in Gli+ Cell-Derived Myofibroblasts Attenuates Fibrosis
Ming Liang et al. J Am Soc Nephrol. 2017 Nov.
Abstract
In damaged kidneys, increased extracellular matrix (ECM) and tissue stiffness stimulate kidney fibrosis through incompletely characterized molecular mechanisms. The transcriptional coactivators yes-associated protein (Yap) and transcriptional coactivator with PDZ-binding motif (Taz) function as mechanosensors in cancer cells and have been implicated in the regulation of myofibroblasts in the kidney. We hypothesized that the development of kidney fibrosis depends on Yap-induced activation and proliferation of kidney fibroblasts. In mice, Yap expression increased in renal fibroblasts after unilateral ureteral obstruction (UUO), in association with worsening of interstitial fibrosis. In cultured fibroblasts, inhibition of Yap/Taz signaling blocked TGF-_β_1-induced fibroblast-to-myofibroblast transformation and ECM production, whereas constitutive activation of Yap promoted fibroblast transformation and ECM production even in the absence of TGF-_β_1. Moreover, in the absence of TGF-_β_1, fibroblasts seeded on a stiffened ECM transformed into myofibroblasts in a process dependent on the activation of Yap. In mice with UUO, the Yap inhibitor verteporfin reduced interstitial fibrosis. Furthermore, Gli1+ cell-specific knockout of Yap/Taz in mice suppressed UUO-induced ECM deposition, myofibroblast accumulation, and interstitial fibrosis. In a UUO-release model, induction of Gli1+ cell-specific Yap/Taz knockout partially reversed the development of interstitial fibrosis. Thus, in the kidney, Yap is a tissue mechanosensor that can be activated by ECM and transforms fibroblasts into myofibroblasts; the interaction of Yap/Taz and ECM forms a feed-forward loop resulting in kidney fibrosis. Identifying mechanisms that interrupt this profibrotic cycle could lead to the development of anti-fibrosis therapy.
Keywords: YAP; fibrosis; obstructive nephropathy; stiffness.
Copyright © 2017 by the American Society of Nephrology.
Figures
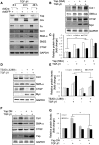
Figure 1.
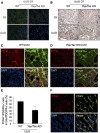
Activated Yap colocalizes with interstitial cells in UUO kidneys. (A) UUO stimulates Yap expression. (B) UUO induces Yap nuclear translocation. After 7 days of UUO, the cytoplasmic and nuclear proteins were prepared and the levels of Yap in kidneys were detected by western blot analysis. GAPDH and histone 1 were used as loading controls for cytoplasm and nuclear, respectively. (C and D) UUO stimulates myofibroblast accumulation and ECM protein deposition. The myofibroblast marker (SMA-α) and ECM proteins were determined by western blot (C) with density analysis shown (D). (E and F) Double immunofluorescent staining of SMA-α with fibronectin (E) or PDGFR_α_ (F) in control and obstructed kidneys after 7 days of UUO. (G and H) Double immunofluorescent staining of Yap in obstructed kidneys with myofibroblast marker SMA-α (G), PDGFR_α_ (H), and inflammatory monocyte marker CD45 (I), respectively (arrowhead indicates Yap staining in interstitial cells and tubules). (J) The percentage of Yap-positive cells in the indicated double-positive cells was counted and calculated. *P<0.05 versus control; _n_=5. CE, cytoplasmic extract; NE, nuclear extract.
Figure 2.
Yap mediates TGF-_β_1–induced fibroblast activation. (A) Mouse fibroblasts were isolated from Yapf/f/Tazf/f mice. The cells were infected with Adeno-Cre; the Adeno-GFP was used as control. After 48 hours, the cells were serum starved and treated with TGF-_β_1 (2 ng/ml), cell lysates were collected at the indicated time points, and the levels of indicated molecules were determined by western blots. (B and C) Mouse fibroblasts expressing constitutive active Yap (5SA) promoted fibroblast transformation into myofibroblasts. Density analysis of the western blots is shown (C). (D and E) Mouse fibroblasts overexpressing dominant negative TEAD (_Δ_289) suppressed the expression of collagen I, SMA-α, and Yap target CTGF (D). The corresponding density analyses of the western blots are shown (E). *P<0.05 versus TGF-_β_1–treated group; _n_=3 repeats. (F) Overexpression of dominant negative Yap (_Δ_60–89) suppressed TGF-_β_1–induced myofibroblast activation. The density analysis of the levels of collagen I and SMA-α are shown in (G). *P<0.05; _n_=3 repeats. AdCre, adenovirus overexpressing Cre; AdGFP, adenovirus overexpressing GFP; DN, dominant negative.
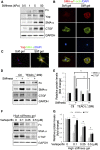
Figure 3.
Matrix stiffness stimulates Yap-dependent fibroblast activation. (A–C) Stiff gel induces Yap expression and fibroblast transformation. Fibroblasts were incubated on polyacrylamide gels of varied stiffness but conjugated with the same collagen concentration on the top surface. This creates a microenvironment in which the mechanical properties are uncoupled from the biochemical properties. The expression of Yap and myofibroblast markers were detected by western blot (A), and the stress fiber formation (B) and Yap localization (C) and their costaining with SMA-α were determined by immunofluorescent staining (C). (D and E) Inhibition of Yap signaling suppresses stiffness-induced fibroblast activation. Control or fibroblasts expressing TEAD (_Δ_289) were seeded on soft or high-stiffness gel for 24 hours, the expression of myofibroblast markers were detected by Western blot (D), and the density was analyzed (E) (P<0.05, compared with stiffness-treated control group). (F and G) Fibroblasts on high-stiffness gel of 50 kPa were incubated with or without varied concentrations of verteporfin for 24 hours, the expression of myofibroblast markers was detected by western blot (F), and the density was analyzed (G). Data were presented from two repeated experiments. P<0.05, compared with control.
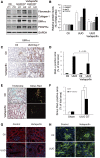
Figure 4.
Yap inhibitor suppresses UUO-induced kidney fibrosis. (A and B) Yap inhibitor suppresses UUO-induced myofibroblast accumulation. Mice with UUO were treated with DMSO or verteporfin (100 mg/kg body wt, i.p. every other day for four times). Markers of myofibroblasts, SMA-α and PDGFR_α_, and ECM proteins, fibronectin, and collagen I, were determined by western blot (A), with density analysis of (A) shown in (B) (*P<0.05 versus UUO group). (C and D) Immunohistochemistry analysis of myofibroblast marker SMA-α (C) was performed and the positive-staining area (D) was measured. (E and F) Kidney fibrosis was determined by trichrome and Sirius Red staining; the area of positive staining, the green color of trichrome staining, and the red color in Sirius red staining were summarized (E). (G and H) The tubule cell marker, E-cadherin, (G) and the endothelial cell marker, VE-cadherin (H), were determined by immunofluorescent staining in control and verteporfin-treated groups. _n_=5; *P<0.05.
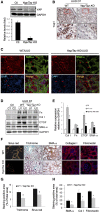
Figure 5.
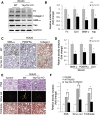
Yap/Taz KO in fibroblasts suppresses renal fibrogenesis. (A) Yap/Taz was knocked out in fibroblasts in Yapf/f/Tazf/f/Gli1-ERCre mice (Yap/Taz KO) after tamoxifen inoculation for 5 days (80 mg/kg body wt). Western blot analysis of Yap expression in fibroblasts from UUO kidneys of control and Yap/Taz KO mice. (B) Immunostaining showed that Yap was absent in interstitial cells of Yap/Taz KO mice. Lower panel represents magnified area of the red frame in the upper panel (arrowhead in panel indicates Yap staining in interstitium in WT). (C) Double immunostaining of Yap and SMA-α in obstructed kidneys of wild-type and Yap/Taz KO mice. Red arrows point to nuclei of interstitial fibroblasts. (D and E) Representative western blot and relative intensity analysis indicate levels of myofibroblast markers in UUO kidneys of WT and Yap/Taz KO mice. (F–H) Sections of obstructed kidneys of WT and Yap/Taz KO mice were immunostained with anti-fibronectin, collagen I, and SMA-α (F). Fibrosis was determined by trichrome and Sirius Red staining (F). The total positive areas were measured and statistically analyzed (G and H) (_n_=5; *P<0.05 versus WT UUO group).
Figure 6.
Yap/Taz KO inhibits the accumulation of proliferating cells in tubulointerstitium after UUO. (A) Yap/Taz KO had no effect on apoptotic cells in obstructed kidneys. Apoptosis was determined by TUNEL analysis; positive cells show green color in the nucleus. (B) Yap/Taz KO inhibits PCNA-positive cells in the tubulointerstitium of obstructed kidney. (C and D) Double immunostaining of PCNA and PDGFR_α_-positive cells in UUO kidney of WT and Yap/Taz KO mice. (E) The percentage of PCNA+/PDGFR_α_+ double-positive cells in total PDGFR_α_+ cells were summarized (_n_=5). (F) CD45 inflammatory cells are positively stained for PCNA in UUO kidney from YAP/Taz KO mice (_n_=5).
Figure 7.
Inducible KO of Yap/Taz reverses UUO-induced kidney fibrosis. (A–D) Release-UUO model was used to determine whether Yap/Taz KO in fibroblasts ameliorates fibrosis. UUO was released after 3 days; Yap/Taz KO mice were induced by adding tamoxifen for 5 days. Wild-type mice that received tamoxifen treatment as Yap/Taz KO mice were taken as control group. After 7 days, kidneys were collected and the expression of myofibroblast markers was detected by western blot (A) and immunohistochemistry (C). The corresponding density analyses are shown (B and D), respectively. (_n_=5; *P<0.05 compared with WT control group). (E and F) Fibrosis was detected by PAS, Sirius Red, and trichrome staining (E) and the positive areas were statistically analyzed (F). (_n_=5; *P<0.05).
Similar articles
- YAP/TAZ Are Mechanoregulators of TGF-_β_-Smad Signaling and Renal Fibrogenesis.
Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, Boo S, Hinz B, Dan Q, Advani A, John R, Wrana JL, Kapus A, Yuen DA. Szeto SG, et al. J Am Soc Nephrol. 2016 Oct;27(10):3117-3128. doi: 10.1681/ASN.2015050499. Epub 2016 Mar 9. J Am Soc Nephrol. 2016. PMID: 26961347 Free PMC article. - Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis.
Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Liu F, et al. Am J Physiol Lung Cell Mol Physiol. 2015 Feb 15;308(4):L344-57. doi: 10.1152/ajplung.00300.2014. Epub 2014 Dec 12. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25502501 Free PMC article. Clinical Trial. - YAP and TAZ are distinct effectors of corneal myofibroblast transformation.
Muppala S, Raghunathan VK, Jalilian I, Thomasy S, Murphy CJ. Muppala S, et al. Exp Eye Res. 2019 Mar;180:102-109. doi: 10.1016/j.exer.2018.12.009. Epub 2018 Dec 19. Exp Eye Res. 2019. PMID: 30578787 Free PMC article. - YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer.
Noguchi S, Saito A, Nagase T. Noguchi S, et al. Int J Mol Sci. 2018 Nov 20;19(11):3674. doi: 10.3390/ijms19113674. Int J Mol Sci. 2018. PMID: 30463366 Free PMC article. Review. - Interplay between YAP/TAZ and Metabolism.
Koo JH, Guan KL. Koo JH, et al. Cell Metab. 2018 Aug 7;28(2):196-206. doi: 10.1016/j.cmet.2018.07.010. Cell Metab. 2018. PMID: 30089241 Review.
Cited by
- The Hippo pathway and its correlation with acute kidney injury.
Zhang C, Li CL, Xu KX, Zheng ZH, Cheng GZ, Wu HJ, Liu J. Zhang C, et al. Zool Res. 2022 Sep 18;43(5):897-910. doi: 10.24272/j.issn.2095-8137.2022.110. Zool Res. 2022. PMID: 36052554 Free PMC article. Review. - SLIT3 deficiency attenuates pressure overload-induced cardiac fibrosis and remodeling.
Gong L, Wang S, Shen L, Liu C, Shenouda M, Li B, Liu X, Shaw JA, Wineman AL, Yang Y, Xiong D, Eichmann A, Evans SM, Weiss SJ, Si MS. Gong L, et al. JCI Insight. 2020 Jun 18;5(12):e136852. doi: 10.1172/jci.insight.136852. JCI Insight. 2020. PMID: 32644051 Free PMC article. - Signaling Mechanisms of Myofibroblastic Activation: Outside-in and Inside-Out.
Zent J, Guo LW. Zent J, et al. Cell Physiol Biochem. 2018;49(3):848-868. doi: 10.1159/000493217. Epub 2018 Sep 5. Cell Physiol Biochem. 2018. PMID: 30184544 Free PMC article. Review. - The role of (pro)renin receptor and its soluble form in cardiovascular diseases.
Wang B, Jie H, Wang S, Dong B, Zou Y. Wang B, et al. Front Cardiovasc Med. 2023 Feb 2;10:1086603. doi: 10.3389/fcvm.2023.1086603. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36824459 Free PMC article. Review. - Adaptations in Hippo-Yap signaling and myofibroblast fate underlie scar-free ear appendage wound healing in spiny mice.
Brewer CM, Nelson BR, Wakenight P, Collins SJ, Okamura DM, Dong XR, Mahoney WM Jr, McKenna A, Shendure J, Timms A, Millen KJ, Majesky MW. Brewer CM, et al. Dev Cell. 2021 Oct 11;56(19):2722-2740.e6. doi: 10.1016/j.devcel.2021.09.008. Epub 2021 Oct 4. Dev Cell. 2021. PMID: 34610329 Free PMC article.
References
- Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ: Diverse origins of the myofibroblast—Implications for kidney fibrosis. Nat Rev Nephrol 11: 233–244, 2015 - PubMed
- Grande MT, López-Novoa JM: Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5: 319–328, 2009 - PubMed
- Rockey DC, Bell PD, Hill JA: Fibrosis--a common pathway to organ injury and failure. N Engl J Med 372: 1138–1149, 2015 - PubMed
MeSH terms
Substances
Grants and funding
- K01 DE026561/DE/NIDCR NIH HHS/United States
- R01 DK095867/DK/NIDDK NIH HHS/United States
- R37 DK037175/DK/NIDDK NIH HHS/United States
- T32 DK007656/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases