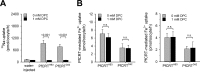Iron is a substrate of the Plasmodium falciparum chloroquine resistance transporter PfCRT in Xenopus oocytes - PubMed (original) (raw)
Iron is a substrate of the Plasmodium falciparum chloroquine resistance transporter PfCRT in Xenopus oocytes
Naziha Bakouh et al. J Biol Chem. 2017.
Abstract
The chloroquine resistance transporter of the human malaria parasite Plasmodium falciparum, PfCRT, is an important determinant of resistance to several quinoline and quinoline-like antimalarial drugs. PfCRT also plays an essential role in the physiology of the parasite during development inside erythrocytes. However, the function of this transporter besides its role in drug resistance is still unclear. Using electrophysiological and flux experiments conducted on PfCRT-expressing Xenopus laevis oocytes, we show here that both wild-type PfCRT and a PfCRT variant associated with chloroquine resistance transport both ferrous and ferric iron, albeit with different kinetics. In particular, we found that the ability to transport ferrous iron is reduced by the specific polymorphisms acquired by the PfCRT variant as a result of chloroquine selection. We further show that iron and chloroquine transport via PfCRT is electrogenic. If these findings in the Xenopus model extend to P. falciparum in vivo, our data suggest that PfCRT might play a role in iron homeostasis, which is essential for the parasite's development in erythrocytes.
Keywords: PfCRT; Xenopus; drug transport; iron; kinetics; malaria; plasmodium.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Figure 1.
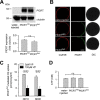
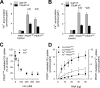
Expression of PfCRT in X. laevis oocytes. A, Western blot analysis of total lysates from oocytes injected with water, PfCRTHB3 cRNA, and PfCRTDd2 cRNA, using a polyclonal guinea pig antiserum specific to PfCRT and a polyclonal rabbit antiserum specific to α-tubulin. A size standard is indicated in kilodaltons. The hybridization signals were subsequently quantified, yielding the expression levels of PfCRTHB3 and PfCRTDd2 relative to the internal standard α-tubulin. The means ± S.E. (error bars) from the number of independent biological replicates NBR = 4 independent Western blot analyses are shown. B, confocal fluorescence images of fixed PfCRTDd2 and PfCRTDd2-expressing oocytes and water-injected control oocytes. Left, fluorescence image of InsP3R, using a specific rabbit antisera and an Alexa Fluor 546 anti-rabbit secondary antibody. Middle, fluorescence image of PfCRT, using a specific guinea pig antiserum and the Alexa Fluor 488 anti-guinea pig secondary antibody. Right, differential interference contrast image. Bar, 250 μm. C, effect of ND10 and ND96 buffer, pH 5.5, on PfCRTDd2-mediated chloroquine uptake in the presence and absence of verapamil (VP; 100 μ
m
). Oocytes were incubated in the respective buffer, pH 7.5, from the moment of cRNA injection. For the flux experiments, chloroquine was added at a final concentration of 50 μ
m
unlabeled chloroquine and 42 n
m
[3H]chloroquine, and the amount of uptake was determined after 60 min of incubation. The means ± S.E. of NBR = 4 independent biological determinations are shown. Statistical significance was assessed using the two-tailed t test. D, intracellular ATP levels of water-injected oocytes and oocytes expressing PfCRTHB3 or PfCRTDd2. Oocytes were incubated in ND10 buffer, pH 7.5, after cRNA injection, for 3 days before the intracellular ATP levels were determined. The mean ± S.E. of NBR = 5 independent biological determinations are shown. The statistical significance was assessed using the Holm–Sidak one-way ANOVA test or a two-tailed t test, where appropriate. n.s., not significant.
Figure 2.
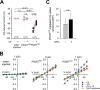
Chloroquine-induced currents in PfCRTDd2-expressing oocytes. A, water-injected oocytes (white circles), PfCRTHB3-expressing oocytes (gray circles), and PfCRTDd2-expressing oocytes (black circles) were voltage-clamped (−50 mV) while superfused with ND10 buffer, pH 6.0. The currents induced upon the addition of 100 μ
m
chloroquine (CQ) were measured before and after supplementation of the medium with 100 μ
m
VP in paired experiments. The lines connecting two data points indicate the response of a single oocyte before and after the treatment. The number of oocytes (n) investigated is indicated above the x axis. The medians are indicated as red lines. The statistical significance was assessed using the Kruskal–Wallis one-way ANOVA on ranks test or the paired t test, where appropriate. The corresponding p values are indicated on the graphs. n.s., not significant. B, current–voltage relationships of water-injected oocytes (left), PfCRTHB3-expressing oocytes (middle), and PfCRTDd2-expressing oocytes (right) were first obtained in ND10 buffer (red-filled circles) and then ND10 buffer containing 100 μ
m
chloroquine (blue-filled circles) before adding 100 μ
m
VP (green-filled inverted triangles). I/V curves upon withdrawal of both compounds are indicated by yellow-filled triangles. Each data point represents the mean ± S.E. (error bars) from n = 15 oocytes. C, PfCRTDd2-mediated chloroquine uptake in the presence and absence of 1 m
m
DPC. PfCRTDd2-expressing oocytes and water-injected oocytes were incubated in ND96 buffer supplemented with and without 1 m
m
DPC and containing 42 n
m
[3H]chloroquine and 50 μ
m
unlabeled chloroquine for 60 min at 25 °C. To calculate the amount of PfCRTDd2-mediated transport, the amount of chloroquine taken up by water-injected oocytes was subtracted. The data represent the means ± S.E. of NBR = 4 independent biological determinations. n.s., not significant according to a t test.
Figure 3.
BYE-induced inward currents in PfCRT-expressing X. laevis oocytes. A and B, water-injected oocytes (white circles), PfCRTHB3-expressing oocytes (gray circles), and PfCRTDd2-expressing oocytes (black circles) were voltage-clamped (−50 mV) while superfused with ND10 buffer, pH 6.0. The currents induced upon the addition of 0.5% BYE were measured before and after supplementation of the medium with 100 μ
m
VP (A) or 100 μ
m
DFO (B) in paired experiments. The number of oocytes investigated is indicated above the x axis. The medians are indicated as red lines. The statistical significance was assessed using the Kruskal–Wallis one-way ANOVA on ranks test or paired t test, where appropriate. The corresponding p values are indicated on the graphs. n.s., not significant.
Figure 4.
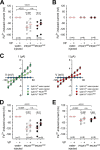
Iron-induced inward currents in PfCRT-expressing X. laevis oocytes. A and B, water-injected oocytes (white circles), PfCRTHB3-expressing oocytes (gray circles), and PfCRTDd2-expressing oocytes (black circles) were voltage-clamped (−50 mV) while superfused with ND10 buffer, pH 6.0. Iron was maintained in divalent form or trivalent form by adding to the buffer a quadruple ratio of
l
-ascorbic acid or nitrilotriacetic acid, respectively. The currents induced by adding 5 μ
m
Fe2+ (A) or 5 μ
m
Fe3+ (B) were measured before and after supplementation of the medium with 100 μ
m
VP. The number (n) of oocytes is indicated above the x axis. The medians are indicated as red lines. The statistical significance was assessed using the Kruskal–Wallis one-way ANOVA on ranks test or paired t test, where appropriate. The corresponding p values are indicated on the graphs. C, current–voltage relationships of water-injected oocytes and PfCRTHB3-expressing oocytes (left) and water-injected oocytes and PfCRTDd2-expressing oocytes (right) were obtained in ND10 buffer, pH 6.0, in the presence and absence of 5 μ
m
ferrous iron. Each data point represents the mean ± S.E. (error bars) of n = 12–15 oocytes. D and E, as in A and B, but this time, a concentration of 1 m
m
ferrous (D) or ferric (E) iron in ND10-mannitol buffer, pH 6.0, was used. n.s., not significant.
Figure 5.
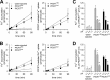
Uptake of ferrous and ferric iron by PfCRT-expressing oocytes. A and B, time courses of ferrous (A) and ferric (B) iron uptake by water-injected oocytes (white circles), PfCRTHB3-expressing oocytes (gray circles), and PfCRTDd2-expressing oocytes (black circles). The uptake assays were performed using the appropriate ND96 buffer, pH 5.5, supplemented with 2 μ
m
radiolabeled 55Fe and 48 μ
m
unlabeled iron. A linear regression was fitted to the data points (_R_2 > 0.9). The right panels show the time courses of PfCRTDd2- and PfCRTHB3-mediated uptake of ferrous and ferric iron after subtracting the uptake in water-injected oocytes from that measured in PfCRTDd2- and PfCRTHB3-expressing oocytes. C and D, pH dependence of ferrous (C) and ferric (D) iron uptake. *, p < 0.05; **, p < 0.01 according to Holm–Sidak one-way ANOVA test. Data were normalized to the amount of iron taken up by PfCRT-expressing oocytes at pH 5.5. The means ± S.E. (error bars) of NBR = 4 independent biological determinations are shown.
Figure 6.
Inhibition of PfCRT-mediated fluxes and cRNA dependence. A and B, effect of verapamil (100 μ
m
) on the uptake of ferrous (A) or ferric (B) iron from an external concentration of 50 μ
m
by water-injected oocytes and PfCRTHB3- and PfCRTDd2-expressing oocytes. Oocytes were analyzed after 60 min of incubation in the respective uptake buffer. The means ± S.E. of NBR = 7–10 independent biological determinations are shown. Statistical significance was evaluated using the two-tailed t test, and the p values are indicated on the graph. C, effect of increasing concentrations of ferrous iron (gray circle) or ferric iron (black circle) on PfCRTDd2-mediated chloroquine uptake. Data were normalized to the amount of PfCRT-mediated chloroquine uptake in the absence of iron. The means ± S.E. of NBR = 3–5 independent biological determinations are shown. D, effect of increasing amounts of cRNA injected on the amount of PfCRT-mediated chloroquine and ferrous iron uptake. Chloroquine and ferrous iron uptake (at the 60-min time point) were measured in parallel assays. The means ± S.E. (error bars) of NBR = 3 independent biological determinations are shown.
Figure 7.
Effect of DPC on Na+, Fe2+, and Fe3+ uptake by PfCRT-expressing oocytes. A, uptake of sodium ions from an external concentration of 22 μ
m
22Na and 5 m
m
unlabeled Na+ by water-injected oocytes and oocytes expressing PfCRTHB3 or PfCRTDd2 in the presence (black bar) and absence (gray bar) of DPC (1 m
m
). The means ± S.E. (error bars) are shown of NBR = 3 independent determinations. Statistical significance was assessed using the two-tailed t test. B, PfCRT-mediated Fe2+ (middle) and Fe3+ (right) by PfCRTHB3- and PfCRTDd2-expressing oocytes in the presence (black bar) and absence (gray bar) of DPC (1 m
m
). Results are presented as means ± S.E. of NBR = 4 independent determinations. n.s., not significant.
Figure 8.
Kinetics of iron uptake by PfCRT-expressing oocytes. A and B, kinetics of PfCRTDd2-mediated (black circles) and PfCRTHB3-mediated uptake (gray circles) of Fe2+ (A) and Fe3+ (B). The uptake of radiolabeled iron into water-injected oocytes and oocytes expressing PfCRTDd2 or PfCRTHB3 was measured in the appropriate ND96 buffer, pH 5.5, containing an extracellular concentration range of 2–400 μ
m
iron (2 μ
m
radiolabeled 55Fe and the appropriate amount of non-radioactive iron). The amount of PfCRT-mediated iron uptake was calculated by subtracting the amount measured in water-injected oocytes from that in oocytes expressing PfCRT at each iron concentration. A least-squares fit of the Michaelis-Menten equation to the resulting data yielded the kinetic parameters compiled in Table 1. The data represent the means ± S.E. (error bars) of NBR = 5 independent biological determinations, with n = 12–30 oocytes per treatment and biological replicate.
Similar articles
- Chloroquine transport via the malaria parasite's chloroquine resistance transporter.
Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Martin RE, et al. Science. 2009 Sep 25;325(5948):1680-2. doi: 10.1126/science.1175667. Science. 2009. PMID: 19779197 - Functional characteristics of the malaria parasite's "chloroquine resistance transporter": implications for chemotherapy.
Summers RL, Martin RE. Summers RL, et al. Virulence. 2010 Jul-Aug;1(4):304-8. doi: 10.4161/viru.1.4.12012. Virulence. 2010. PMID: 21178460 - Verapamil-Sensitive Transport of Quinacrine and Methylene Blue via the Plasmodium falciparum Chloroquine Resistance Transporter Reduces the Parasite's Susceptibility to these Tricyclic Drugs.
van Schalkwyk DA, Nash MN, Shafik SH, Summers RL, Lehane AM, Smith PJ, Martin RE. van Schalkwyk DA, et al. J Infect Dis. 2016 Mar 1;213(5):800-10. doi: 10.1093/infdis/jiv509. Epub 2015 Oct 26. J Infect Dis. 2016. PMID: 26503982 - PfCRT-mediated drug transport in malarial parasites.
Roepe PD. Roepe PD. Biochemistry. 2011 Jan 18;50(2):163-71. doi: 10.1021/bi101638n. Epub 2010 Dec 22. Biochemistry. 2011. PMID: 21142008 Free PMC article. Review. - Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance.
Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. Bray PG, et al. Mol Microbiol. 2005 Apr;56(2):323-33. doi: 10.1111/j.1365-2958.2005.04556.x. Mol Microbiol. 2005. PMID: 15813727 Review.
Cited by
- The Toxoplasma plant-like vacuolar compartment (PLVAC).
Stasic AJ, Moreno SNJ, Carruthers VB, Dou Z. Stasic AJ, et al. J Eukaryot Microbiol. 2022 Nov;69(6):e12951. doi: 10.1111/jeu.12951. Epub 2022 Oct 27. J Eukaryot Microbiol. 2022. PMID: 36218001 Free PMC article. Review. - The Knock-Down of the Chloroquine Resistance Transporter PfCRT Is Linked to Oligopeptide Handling in Plasmodium falciparum.
Sanchez CP, Manson EDT, Moliner Cubel S, Mandel L, Weidt SK, Barrett MP, Lanzer M. Sanchez CP, et al. Microbiol Spectr. 2022 Aug 31;10(4):e0110122. doi: 10.1128/spectrum.01101-22. Epub 2022 Jul 18. Microbiol Spectr. 2022. PMID: 35867395 Free PMC article. - Consistent signatures of selection from genomic analysis of pairs of temporal and spatial Plasmodium falciparum populations from The Gambia.
Amambua-Ngwa A, Jeffries D, Amato R, Worwui A, Karim M, Ceesay S, Nyang H, Nwakanma D, Okebe J, Kwiatkowski D, Conway DJ, D'Alessandro U. Amambua-Ngwa A, et al. Sci Rep. 2018 Jun 26;8(1):9687. doi: 10.1038/s41598-018-28017-5. Sci Rep. 2018. PMID: 29946063 Free PMC article. - Phosphomimetic substitution at Ser-33 of the chloroquine resistance transporter PfCRT reconstitutes drug responses in Plasmodium falciparum.
Sanchez CP, Moliner Cubel S, Nyboer B, Jankowska-Döllken M, Schaeffer-Reiss C, Ayoub D, Planelles G, Lanzer M. Sanchez CP, et al. J Biol Chem. 2019 Aug 23;294(34):12766-12778. doi: 10.1074/jbc.RA119.009464. Epub 2019 Jul 8. J Biol Chem. 2019. PMID: 31285265 Free PMC article. - Structural and evolutionary analyses of the Plasmodium falciparum chloroquine resistance transporter.
Coppée R, Sabbagh A, Clain J. Coppée R, et al. Sci Rep. 2020 Mar 16;10(1):4842. doi: 10.1038/s41598-020-61181-1. Sci Rep. 2020. PMID: 32179795 Free PMC article.
References
- Fidock D. A., Nomura T., Talley A. K., Cooper R. A., Dzekunov S. M., Ferdig M. T., Ursos L. M., Sidhu A. B., Naudé B., Deitsch K. W., Su X. Z., Wootton J. C., Roepe P. D., and Wellems T. E. (2000) Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6, 861–871 - PMC - PubMed
- Martin R. E., Marchetti R. V., Cowan A. I., Howitt S. M., Bröer S., and Kirk K. (2009) Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science 325, 1680–1682 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical