Silencing Neurons: Tools, Applications, and Experimental Constraints - PubMed (original) (raw)
Review
Silencing Neurons: Tools, Applications, and Experimental Constraints
J Simon Wiegert et al. Neuron. 2017.
Abstract
Reversible silencing of neuronal activity is a powerful approach for isolating the roles of specific neuronal populations in circuit dynamics and behavior. In contrast with neuronal excitation, for which the majority of studies have used a limited number of optogenetic and chemogenetic tools, the number of genetically encoded tools used for inhibition of neuronal activity has vastly expanded. Silencing strategies vary widely in their mechanism of action and in their spatial and temporal scales. Although such manipulations are commonly applied, the design and interpretation of neuronal silencing experiments present unique challenges, both technically and conceptually. Here, we review the most commonly used tools for silencing neuronal activity and provide an in-depth analysis of their mechanism of action and utility for particular experimental applications. We further discuss the considerations that need to be given to experimental design, analysis, and interpretation of collected data. Finally, we discuss future directions for the development of new silencing approaches in neuroscience.
Keywords: archaerhodopsin; channelrhodopsin; chemogenetics; halorhodopsin; light-activated G-protein-coupled receptors; light-gated anion channelrhodopsins; neuronal silencing; optogenetics; synaptic transmission.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Figure 1. Silencing Strategies in Neurons
(A) Silencing strategies are depicted with regard to reversibility, cellular specificity, and spatiotemporal precision. Principal neurons are shown with axonal projections terminating in two distinct target regions. Silenced neurons or subcellular compartments are red. Unaffected neurons or subcellular compartments are gray. Neurons expressing a genetically encoded silencing tool (small circular symbol) are blue where they are not silenced. Target regions are circled red when silencing effect is specific to terminals. (B) Temporal precision of onset (left) and offset (right) versus spatial precision of silencing effect mediated by various strategies. Note the logarithmic scaling of the axes. Colors indicate different classes of tools or strategies.
Figure 2. Genetically Encoded Silencing Tools
(A) Overview of genetically encoded silencing tools, grouped according to their mode of activation. The group of light-activated silencing tools comprises rhodopsin ion pumps (1–2), anion channelrhodopsins (ACRs) (3), LOV-domain-activated potassium channels (4), ion channels coupled to photoswitched tethered ligands (PTL) (5), potassium channels downstream of G-protein-coupled receptor (GPCR) signaling (6), and the synaptic vesicle proteins VAMP-2 (blue) or synaptophysin-1 (violet) (7). In the latter case, chromophore-assisted light inactivation (CALI) of those proteins is achieved via singlet-oxygen generation through miniSOG. The ligand-activated tools consist of GPCRs (8) and chloride channels activated by highly selective, otherwise inert ligands (9). Although GPCRs mainly act through G-protein-coupled inward-rectifying potassium (GIRK) channels, they also recruit other mechanisms (see Main Text). The third group consists of tools that are permanently active when expressed (10–12). (B) Approximate action spectra of light-activated silencing tools. Hemoglobin light absorption (μ10) and gray matter scattering drastically decrease toward longer wavelengths. Values for hemoglobin absorption from:
http://omlc.org/spectra/hemoglobin/summary.html
. Gray matter scattering was estimated using the following function: 2.37*(λ/500 nm)−1.15 (from Liu et al., 2015). Below are action spectra of the indicated tools. Color saturation of the horizontal bars scales approximately with relative absorption. * The action spectrum of vLWO was maximal at the highest wavelength tested and is likely to extend farther into the near-infrared range.
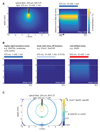
Figure 3. Monte Carlo Simulation of Light-Induced Heat Distribution and Light Propagation in Neuronal Tissue
(A) Simulation of tissue heating in a “typical” in vivo optogenetic experiment using continuous blue light for ChR2 activation. Tissue heating is modeled for optical stimulation with 470 nm light for 1 min through a fiber with 200 μm diameter and a numerical aperture of 0.37. Left: spatial heat distribution in 2D cross-section under the optical fiber. Right: average temperature change over time within a radial distance of 0.2 mm from the fiber tip. The location of the fiber tip is indicated by the white dashed line. (B) Strategies to reduce tissue heating in optogenetic silencing experiments. Left: various optogenetic silencing tools are orders of magnitudes more light sensitive than the classical rhodopsin ion pumps NpHR or Arch. Therefore, lower light intensities can be used to silence neurons within a given volume. Middle: silencing tools with slow off/closing kinetics can sustain their silencing activity after light is off. Using short light pulses of high intensities avoids heat buildup. Right: long-wavelength light is absorbed less strongly in brain tissue and can be used to activate red-shifted silencing tools. Scales are as in (A). (C) Monte Carlo simulation of light penetration under illumination conditions considered not to increase spiking activity due to tissue heating (see Stujenske et al., 2015). Colored contours circumcise volumes where photon flux has dropped 10-fold starting at 10 mW/mm2. 530 nm light can be used to activate various opsins such as Arch, NpHR, optoXRs, GtACR1, or vLWO. Required photon flux to achieve maximal silencing is several orders of magnitude lower for GtACR1 or vLWO compared to the other opsins. Thus, large volumes can be efficiently silenced with such highly sensitive tools without significantly heating the tissue.
Figure 4. Compartment-Specific Action of Optogenetic and Chemogenetic Silencing Tools
(A) Schematic depiction of an archetypical neuron. Color indicates intracellular chloride concentration, from lower (black) to higher (red) chloride concentration than in the soma (blue, Kole and Stuart, 2012). (B) ACR activation throughout the neuron leads to light-activated chloride conductance, which is predominantly hyperpolarizing in soma and dendrites, but could be depolarizing in the axon due to an elevated intra-axonal chloride concentration (Mahn et al., 2016; Malyshev et al., 2017). (C) Halorhodopsins such as eNpHR3.0 and Jaws carry chloride into the cytoplasm uniformly across the axo-dendritic gradient due to their active ion-pumping function. While these tools can effectively silence both somatic and axonal activity, they can also lead to an increase in the chloride concentration and result in a depolarizing effect of GABAA receptors (Raimondo et al., 2012). (D) Proton-pumping rhodopsins hyperpolarize the neuron through active proton pumping but might also cause drastic changes in pH and elevated intracellular Ca2+, which in the synaptic terminals could lead to increased spontaneous release of neurotransmitter (Mahn et al., 2016). (E) Gi/o-coupled vertebrate rhodopsins inhibit the somatodendritic compartment through recruitment of GIRK channels (Masseck et al., 2014). These rhodopsins were reported to inhibit neurotransmitter release, potentially through direct action on Ca2+ channels (Li et al., 2005). (F) Gi/o-coupled DREADDs inhibit the somatodendritic compartment through recruitment of GIRK channels. Inhibition of synaptic transmission is probably GIRK independent but rather occurs through G-protein-mediated silencing of Ca2+ channel activity (Stachniak et al., 2014; Vardy et al., 2015).
Similar articles
- Microbial Rhodopsin Optogenetic Tools: Application for Analyses of Synaptic Transmission and of Neuronal Network Activity in Behavior.
Glock C, Nagpal J, Gottschalk A. Glock C, et al. Methods Mol Biol. 2015;1327:87-103. doi: 10.1007/978-1-4939-2842-2_8. Methods Mol Biol. 2015. PMID: 26423970 - Optogenetic inhibition of behavior with anion channelrhodopsins.
Mohammad F, Stewart JC, Ott S, Chlebikova K, Chua JY, Koh TW, Ho J, Claridge-Chang A. Mohammad F, et al. Nat Methods. 2017 Mar;14(3):271-274. doi: 10.1038/nmeth.4148. Epub 2017 Jan 23. Nat Methods. 2017. PMID: 28114289 - High-efficiency optogenetic silencing with soma-targeted anion-conducting channelrhodopsins.
Mahn M, Gibor L, Patil P, Cohen-Kashi Malina K, Oring S, Printz Y, Levy R, Lampl I, Yizhar O. Mahn M, et al. Nat Commun. 2018 Oct 8;9(1):4125. doi: 10.1038/s41467-018-06511-8. Nat Commun. 2018. PMID: 30297821 Free PMC article. - Development of transgenic animals for optogenetic manipulation of mammalian nervous system function: progress and prospects for behavioral neuroscience.
Ting JT, Feng G. Ting JT, et al. Behav Brain Res. 2013 Oct 15;255:3-18. doi: 10.1016/j.bbr.2013.02.037. Epub 2013 Mar 6. Behav Brain Res. 2013. PMID: 23473879 Free PMC article. Review. - Optogenetics and pharmacogenetics: principles and applications.
Jiang J, Cui H, Rahmouni K. Jiang J, et al. Am J Physiol Regul Integr Comp Physiol. 2017 Dec 1;313(6):R633-R645. doi: 10.1152/ajpregu.00091.2017. Epub 2017 Aug 9. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28794102 Free PMC article. Review.
Cited by
- A hypothalamic circuit mechanism underlying the impact of stress on memory and sleep.
Wiest A, Maurer JJ, Weber F, Chung S. Wiest A, et al. bioRxiv [Preprint]. 2024 Oct 18:2024.10.17.618467. doi: 10.1101/2024.10.17.618467. bioRxiv. 2024. PMID: 39463948 Free PMC article. Preprint. - All-optical reporting of inhibitory receptor driving force in the nervous system.
Selfe JS, Steyn TJS, Shorer EF, Burman RJ, Düsterwald KM, Kraitzick AZ, Abdelfattah AS, Schreiter ER, Newey SE, Akerman CJ, Raimondo JV. Selfe JS, et al. Nat Commun. 2024 Oct 16;15(1):8913. doi: 10.1038/s41467-024-53074-y. Nat Commun. 2024. PMID: 39414774 Free PMC article. - Bridging model and experiment in systems neuroscience with Cleo: the Closed-Loop, Electrophysiology, and Optophysiology simulation testbed.
Johnsen KA, Cruzado NA, Menard ZC, Willats AA, Charles AS, Markowitz JE, Rozell CJ. Johnsen KA, et al. bioRxiv [Preprint]. 2024 Jul 9:2023.01.27.525963. doi: 10.1101/2023.01.27.525963. bioRxiv. 2024. PMID: 39026717 Free PMC article. Preprint. - Pulsatile electrical stimulation creates predictable, correctable disruptions in neural firing.
Steinhardt CR, Mitchell DE, Cullen KE, Fridman GY. Steinhardt CR, et al. Nat Commun. 2024 Jul 12;15(1):5861. doi: 10.1038/s41467-024-49900-y. Nat Commun. 2024. PMID: 38997274 Free PMC article. - Fiber-based Probes for Electrophysiology, Photometry, Optical and Electrical Stimulation, Drug Delivery, and Fast-Scan Cyclic Voltammetry In Vivo.
Driscoll N, Antonini MJ, Cannon TM, Maretich P, Olaitan G, Phi Van VD, Nagao K, Sahasrabudhe A, Vargas E, Hunt S, Hummel M, Mupparaju S, Jasanoff A, Venton J, Anikeeva P. Driscoll N, et al. bioRxiv [Preprint]. 2024 Jun 8:2024.06.07.598004. doi: 10.1101/2024.06.07.598004. bioRxiv. 2024. PMID: 38895451 Free PMC article. Preprint.
References
- Abraham WC. Metaplasticity: Tuning synapses and networks for plasticity. Nat Rev Neurosci. 2008;9:387. - PubMed
- Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources