SLC39A4 expression is associated with enhanced cell migration, cisplatin resistance, and poor survival in non-small cell lung cancer - PubMed (original) (raw)
SLC39A4 expression is associated with enhanced cell migration, cisplatin resistance, and poor survival in non-small cell lung cancer
Dong-Ming Wu et al. Sci Rep. 2017.
Abstract
The zinc transporter SLC39A4 influences epithelial cell morphology and migration in various cancers; however, its role in regulating cell invasion and chemotherapeutic resistance in human lung cancer is not yet clear. Here, integrated analysis of gene expression in non-small cell lung cancer revealed that SLC39A4 expression is significantly correlated with increased tumour size and regional lymph node spread, as well as shorter overall survival (OS) and disease-free survival (DFS). SLC39A4 silencing by lentivirus-mediated shRNA blocked human lung cancer cell epithelial-mesenchymal transition and metastasis in vitro and in vivo, respectively. Moreover, SLC39A4 knockdown enhanced cancer cell sensitivity to cisplatin-induced death by inhibiting stemness in lung cancer cells. Collectively, these data suggest that SLC39A4 may be a novel therapeutic target and predictive marker of tumour metastasis in non-small cell lung cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
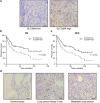
SLC39A4 is an independent predictor of non-small cell lung cancer (NSCLC). (a) Meta-analysis of SLC39A4 expression in NSCLC samples and normal tissues. Mean differences were estimated using an inverse variance (IV)-weighted random-effects model (Mean difference, 1.48; 95% CI, 0.88–2.08). (b) Meta-analysis of SLC39A4 specificity and sensitivity as a biomarker of NSCLC. (c) SLC39A4 expression association with tumour stage using a Mantel-Haenszel (M-H)-weighted random-effects model (Odds Ratio [OR], 0.52; 95% CI, 0.34–0.80).
Figure 2
Kaplan-Meier analysis of association of SLC39A4 expression with patient survival. (a) Representative images of SLC39A4 low and SLC39A4 high samples (scale bar, 50 μm). (b,c) Association between SLC39A4 expression and overall survival (OS, b) and disease-free survival (DFS, c) by Kaplan-Meier analysis. (d) SLC39A4 expression in normal tissue, lung cancer in situ, and metastatic lesions (scale bar, 50 μm).
Figure 3
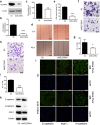
SLC39A4 silencing inhibits A549 cells metastasis in vitro. (a,b) SLC39A4 expression in A549 knockdown and empty vector control (Ctl) cells by western blot analysis (a) and qPCR (b,c). Zn2+ concentration in A549 cells after knockdown of SLC39A4 in vivo. (d,e) Analysis of SLC39A4 knockdown and Ctl cell migration in wound-healing assays (scale bar, 500 μm). Representative images (d) and quantitation (e) are shown. (f,g) Cell migration was monitored in transwell assays with SLC39A4 knockdown and Ctl A549 cells (scale bar, 100 μm). Representative images (f) and quantitation (g) are shown. (h,i) Cell invasion was monitored in matrigel transwell assays with SLC39A4 knockdown and Ctl A549 cells (scale bar, 100 μm). Representative images (h) and quantitation (i) are shown. (j,k) Analysis of E-cadherin (epithelial marker) and FSP-1 and N-cadherin (mesenchymal markers) expression in knockdown and control cells by immunofluorescence staining (j) and western blotting (k) (scale bar, 50 μm).
Figure 4
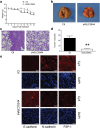
SLC39A4 silencing limits metastatic spread in a mouse model of lung cancer. (a) Body weight measurements of tumour-bearing mice. (b) Representative images of mouse lungs 30 d after shSLC39A4 or Ctl A549 cell engraftment. (c) Representative images of H&E staining in lung tissue sections from each group (scale bar, 100 μm). (d) The average number of metastatic tumour nodules in the lungs xenograft mice. E. Immunofluorescence for E-cadherin, FSP-1, and N-cadherin expression in A549 shSLC39A4 or Ctl subcutaneous tumours (scale bar, 100 μm).
Figure 5
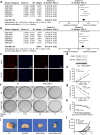
Inhibition of SLC39A4 enhances cisplatin resistance in human NSCLC cells. (a) Correlation of SLC39A4 and CD44 expression, Fisher’s Z = 0.26 (95% CI, 0.19–0.33). (b) Correlation of SLC39A4 and CD133 expression, Fisher’s Z = 0.21 (95% CI, 0.14–0.28). (c,d) Analysis of CD44 and CD133 expression by immunofluorescence (c) and western blotting (d) in shSLC39A4 or Ctl A549 cells (scale bar, 50 μm). (e,f) Cisplatin-induced cell death was monitored in CCK-8 arrays (e) and colony formation assays (f,g). Quantitation of colony formation. (h–j) Body weight (h) and tumour size (i,j) were used to examine cisplatin sensitivity in shSLC39A4 and Ctl A549 tumour-bearing mice.
Similar articles
- Impact of Brachyury on epithelial-mesenchymal transitions and chemosensitivity in non-small cell lung cancer.
Xu K, Liu B, Liu Y. Xu K, et al. Mol Med Rep. 2015 Jul;12(1):995-1001. doi: 10.3892/mmr.2015.3348. Epub 2015 Feb 13. Mol Med Rep. 2015. PMID: 25683840 Free PMC article. - BCL9 promotes epithelial mesenchymal transition and invasion in cisplatin resistant NSCLC cells via β-catenin pathway.
Zhang Y, Zhang Q, Chen H, Wang C. Zhang Y, et al. Life Sci. 2018 Sep 1;208:284-294. doi: 10.1016/j.lfs.2018.07.023. Epub 2018 Jul 23. Life Sci. 2018. PMID: 30009824 - Expression of Ras-related protein 25 predicts chemotherapy resistance and prognosis in advanced non-small cell lung cancer.
Ma YF, Yang B, Li J, Zhang T, Guo JT, Chen L, Li M, Chu J, Liang CY, Liu Y. Ma YF, et al. Genet Mol Res. 2015 Oct 30;14(4):13998-4008. doi: 10.4238/2015.October.29.19. Genet Mol Res. 2015. PMID: 26535714 - Pharmacogenomics of cisplatin sensitivity in non-small cell lung cancer.
Rose MC, Kostyanovskaya E, Huang RS. Rose MC, et al. Genomics Proteomics Bioinformatics. 2014 Oct;12(5):198-209. doi: 10.1016/j.gpb.2014.10.003. Epub 2014 Oct 28. Genomics Proteomics Bioinformatics. 2014. PMID: 25449594 Free PMC article. Review.
Cited by
- A Comprehensive Prognostic and Immunological Analysis of a Six-Gene Signature Associated With Glycolysis and Immune Response in Uveal Melanoma.
Liu J, Lu J, Li W. Liu J, et al. Front Immunol. 2021 Sep 22;12:738068. doi: 10.3389/fimmu.2021.738068. eCollection 2021. Front Immunol. 2021. PMID: 34630418 Free PMC article. - Transparent sparse graph pathway network for analyzing the internal relationship of lung cancer.
Jin Z, Shi Y, Zhou L. Jin Z, et al. Front Genet. 2024 Oct 1;15:1437174. doi: 10.3389/fgene.2024.1437174. eCollection 2024. Front Genet. 2024. PMID: 39411374 Free PMC article. - The ZIP6/ZIP10 heteromer is essential for the zinc-mediated trigger of mitosis.
Nimmanon T, Ziliotto S, Ogle O, Burt A, Gee JMW, Andrews GK, Kille P, Hogstrand C, Maret W, Taylor KM. Nimmanon T, et al. Cell Mol Life Sci. 2021 Feb;78(4):1781-1798. doi: 10.1007/s00018-020-03616-6. Epub 2020 Aug 14. Cell Mol Life Sci. 2021. PMID: 32797246 Free PMC article. - Knockdown of SLC39A4 Expression Inhibits the Proliferation and Motility of Gallbladder Cancer Cells and Tumor Formation in Nude Mice.
Li M, Fan K, Zheng B, Zekria D, Suo T, Liu H, Shen S, Liu H, Ni X. Li M, et al. Cancer Manag Res. 2021 Mar 8;13:2235-2246. doi: 10.2147/CMAR.S282269. eCollection 2021. Cancer Manag Res. 2021. PMID: 33727860 Free PMC article. - Zinc Ions Modulate YY1 Activity: Relevance in Carcinogenesis.
Figiel M, Górka AK, Górecki A. Figiel M, et al. Cancers (Basel). 2023 Aug 30;15(17):4338. doi: 10.3390/cancers15174338. Cancers (Basel). 2023. PMID: 37686614 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical