Human dendritic cells activated with MV130 induce Th1, Th17 and IL-10 responses via RIPK2 and MyD88 signalling pathways - PubMed (original) (raw)
. 2018 Jan;48(1):180-193.
doi: 10.1002/eji.201747024. Epub 2017 Sep 14.
Cristina Benito-Villalvilla 1, Silvia Sánchez-Ramón 2 3, Sofía Sirvent 1, Carmen M Diez-Rivero 4, Laura Conejero 5, Paola Brandi 5, Lourdes Hernández-Cillero 2 6, Juliana Lucía Ochoa 2, Beatriz Pérez-Villamil 6, David Sancho 5, José Luis Subiza 2 3 4, Oscar Palomares 1
Affiliations
- PMID: 28799230
- PMCID: PMC5813220
- DOI: 10.1002/eji.201747024
Human dendritic cells activated with MV130 induce Th1, Th17 and IL-10 responses via RIPK2 and MyD88 signalling pathways
Cristina Cirauqui et al. Eur J Immunol. 2018 Jan.
Abstract
Recurrent respiratory tract infections (RRTIs) are the first leading cause of community- and nosocomial-acquired infections. Antibiotics remain the mainstay of treatment, enhancing the potential to develop antibiotic resistances. Therefore, the development of new alternative approaches to prevent and treat RRTIs is highly demanded. Daily sublingual administration of the whole heat-inactivated polybacterial preparation (PBP) MV130 significantly reduced the rate of respiratory infections in RRTIs patients, however, the immunological mechanisms of action remain unknown. Herein, we study the capacity of MV130 to immunomodulate the function of human dendritic cells (DCs) as a potential mechanism that contribute to the clinical benefits. We demonstrate that DCs from RRTIs patients and healthy controls display similar ex vivo immunological responses to MV130. By combining systems biology and functional immunological approaches we show that MV130 promotes the generation of Th1/Th17 responses via receptor-interacting serine/threonine-protein kinase-2 (RIPK2)- and myeloid-differentiation primary-response gene-88 (MyD88)-mediated signalling pathways under the control of IL-10. In vivo BALB/c mice sublingually immunized with MV130 display potent systemic Th1/Th17 and IL-10 responses against related and unrelated antigens. We elucidate immunological mechanisms underlying the potential way of action of MV130, which might help to design alternative treatments in other clinical conditions with high risk of recurrent infections.
Keywords: Dendritic cells (DCs); IL-10-producing T cells; Recurrent respiratory tract infections (RRTIs); Th1/Th17 cells; Whole heat-inactivated polybacterial vaccines.
© 2017 The Authors. European Journal of Immunology published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Figure 1
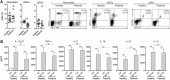
MV130‐activated hmoDCs from healthy subjects and RRTIs patients produce pro‐inflammatory cytokines with high levels of IL‐10. (A) Percentage of monocytes (HLA‐DR+ CD14+), mDCs (HLA‐DR+ CD19− CD1c+ CD11c+) and pDCs (HLA‐DR+ CD123+ CD303+) in freshly isolated PBMCs from healthy donors (n = 9) and RRTI patients (n = 9). Data are pooled from three independent experiments with three donor and three patient samples per experiment. Representative dot plots showing the gated cells are displayed on the right side. (B) Cytokines levels in cell‐free supernatants (IL‐12p70, TNF‐α, IL‐6, IL‐β, IL‐23, and IL‐10) quantified by ELISA after stimulation of hmoDCs from healthy subjects (n = 9) and patients (n = 9) with Ctrl (control containing all excipients except the bacteria) or MV130 for 24 h. All data are represented as mean ± S.E.M. Unpaired (for A) and paired (for B) _t_‐test, *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 2
MV130‐activated hmoDCs from healthy donors and RRTIs patients induce T cell proliferation and the generation of Th1, Th17 and IL‐10‐producing T cells. (A) Representative dot plots of proliferating CFSE‐labelled allogeneic naïve CD4+ T cells after 3 days of co‐culture with hmoDCs from healthy subjects (n = 5) and patients (n = 5) in the presence of Ctrl or MV130. The frequency of proliferating cells is displayed inside the plot. (B) ELISA quantification of IFN‐γ, IL‐17A, IL‐5 and IL‐10 cytokines in cell free supernatants produced by allogeneic naïve CD4+ T cells primed by Ctrl‐ or MV130‐activated hmoDCs from healthy subjects (n = 9) and patients (n = 9) after 3 days. (C) Percentage of CD3+CD4+ T cells producing IFN‐γ, IL‐17A, IL‐4 and IL‐10 generated after 3 days of co‐culture of Ctrl‐ or MV130‐activated hmoDCs from healthy donors and allogeneic CD4+ T cells as determined by intracellular staining (n = 4–7). Representative dot plots are shown for each cytokine. (D) Percentage of CD3+CD4+ T cells simultaneously producing IL‐10 and IFN‐γ, IL‐10 and IL‐17A, or IL‐17A and IFN‐γ after intracellular staining as determined by flow cytometry analysis (n = 5). Panels B, C and D. Data are pooled from 3 to 5 independent experiments. Results are mean ± S.E.M. Wilcoxon‐signed‐rank test *p < 0.05; **p < 0.01.
Figure 3
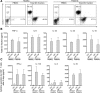
MV130‐activated total blood DCs from healthy subjects and RRTIs patients produce pro‐ and anti‐inflammatory cytokines and induce Th1, Th17 and IL‐10‐producing T cells. (A) Representative dot plots of the different DC subsets contained in PBMCs from healthy subjects and RRTIs patients and the obtained enriched total DC fraction in each case: pDCs (HLA‐DR+ CD19− CD303+); mDCs (HLA‐DR+ CD19− CD1c+). The percentage for each DC subset is displayed inside the quadrants. Data are representative of three independent experiments. (B) Cytokine production by the enriched total DC fraction from healthy individuals and patients after 24 h of stimulation with Ctrl or MV130 quantified by ELISA. Data are pooled from three independent experiments (C) Cytokines produced by allogeneic naïve CD4+ T cells primed by control‐ or MV130‐activated total DCs from healthy subjects and patients after 3 days quantified by ELISA. Results are the mean ± S.E.M. of three independent experiments.
Figure 4
Enriched mDCs produce proinflammatory cytokines and IL‐10 after MV130 stimulation whereas enriched pDCs only IL‐6. (A) Representative dot plots for mDCs contained in PBMCs and the obtained enriched mDCs (HLA‐DR+ CD19− CD1c+ CD11c+). (B) Cytokine production by the enriched mDCs after 24 h of stimulation with Ctrl or MV130 quantified by ELISA. (C) Representative dot plots for pDCs contained in PBMCs and the obtained enriched pDCs (HLA‐DR+ CD123+ CD303+). (D) Cytokines produced by the enriched pDCs after 24 h of stimulation with Ctrl or MV130 quantified by ELISA. Panels B and D: Data are pooled from 3 independent experiments. (E) IFN‐α production by enriched pDCs stimulated for 24 h with Ctrl, MV130 or TLR9‐ligand as positive control. Results are the mean ± S.E.M. of three independent experiments.
Figure 5
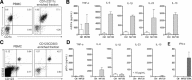
Global comparative transcriptome analysis by DNA microarrays of hmoDCs treated with control or MV130 for 24 h. (A) The Volcano plot depicts −log10 of corrected p value versus log2 of fold‐change (FC) for each gene. Moderate _t_‐test analysis was used for n = 4 independent experiments to find 1456 genes differentially expressed (FC > 2 and p < 0.05) between control and treated samples. Up‐ and down‐regulated genes are highlighted in red and blue, respectively. (B) GO term analysis using GeneCodis software was performed for the up‐ and down‐regulated genes. Altered GO terms at _p_ < 0.05 and > 10 genes are displayed in red and blue chart diagrams for up‐ and down‐regulated genes, respectively. (C) KEGG analysis of the 10 altered (p < 0.06 and > 10 genes) immunoregulatory pathways analyzed with DAVID software according to the −log10 of corrected p value and the number of genes involved in each pathway. (D) Protein interaction network using as input the 49 non‐redundant genes from JAK‐STAT, NLR and TLR signalling pathways with the STRING program at confidence of ≥ 0.7. The main clusters of associated genes identified by the predicted network are indicated. Key molecules for each cluster are highlighted. The connected lines represent the associations according to the color code indicated in the figure. Data are pooled from 4 independent experiments.
Figure 6
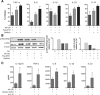
MV130 immunomodulates human DCs’ function by mechanisms depending on NLR/RIPK2‐ and TLR/MyD88‐mediated signaling pathways under the control of IL‐10. (A) The graphs display the percentage of inhibition of TNF‐α, IL‐6, IL‐1β, IL‐23 and IL‐10 production by MV130‐treated hmoDCs from healthy donors by the indicated inhibitors (Pepinh‐MYD and/or Gefitinb) related to vehicle controls (Pepinh‐Control and/or DMSO). Results are pooled from 6–8 independent experiments. Mean ± S.E.M. is shown. Wilcoxon‐signed‐rank test *p < 0.05. (B) Western blot analysis of protein extracts from hmoDCs stimulated for 30 min under the indicated conditions (Ctrl, MV130, Pepinh‐MYD or Gefitinb) and quantification of the reactive phosphorylated bands by scanning densitometry. Graphs in the right side represent the quantification of the corresponding reactive bands (phospho‐IKKα/IKKβ (Ser176/Ser177) and phospho‐IkBα (Ser32/36)) with respect to the β‐actin and relative to Ctrl‐treated condition for each case. One representative example out of 3 is shown. (C) Increase of IL‐12p70, TNF‐α, IL‐6, IL‐β and IL‐23 cytokine production by MV130‐activated hmoDCs in the presence of specific blocking antibodies against IL‐10 (α‐IL‐10) with respect to the levels in the presence of isotype control. Results are pooled from six independent experiments. Mean ± S.E.M. is shown. Wilcoxon‐ signed‐rank test *p < 0.05.
Figure 7
Induction of Th1, Th17 and IL‐10 immune responses after in vivo MV130 sublingual immunization of BALB/c mice. (A) Scheme of the sublingual immunization protocol and analysis of induced systemic responses. (B) Proliferation of CFSE‐labelled CD4+ T cells from splenocytes isolated from mice immunized sublingually with MV130 or control after in vitro stimulation with MV130 or control. (C) Cytokine production (IFN‐γ, IL‐17A and IL‐10) by splenocytes isolated from mice immunized sublingually with MV130 or control and stimulated in vitro with MV130 or control. (D) Scheme of the immunization/challenge protocol followed to assess immune responses against the unrelated antigen OVA. (E) Proliferation of CFSE‐labelled CD4+ T cells from splenocytes isolated from the indicated mice after in vitro stimulation with OVA or control. (C) Cytokine production by the indicated splenocytes stimulated in vitro with OVA or control. Results are mean ± S.E.M. of n = 6 (B), n = 3 (C), n = 8 (E) and n = 6 (F) from two independent experiments. Ctrl, control containing all excipients except the bacteria; MV130, PBP Bactek. Unpaired or paired t test, *p < 0.05; **p < 0.01; ***p < 0.001.
Similar articles
- MV140, a sublingual polyvalent bacterial preparation to treat recurrent urinary tract infections, licenses human dendritic cells for generating Th1, Th17, and IL-10 responses via Syk and MyD88.
Benito-Villalvilla C, Cirauqui C, Diez-Rivero CM, Casanovas M, Subiza JL, Palomares O. Benito-Villalvilla C, et al. Mucosal Immunol. 2017 Jul;10(4):924-935. doi: 10.1038/mi.2016.112. Epub 2016 Dec 14. Mucosal Immunol. 2017. PMID: 27966556 - Trained Immunity-Based Vaccine in B Cell Hematological Malignancies With Recurrent Infections: A New Therapeutic Approach.
Ochoa-Grullón J, Benavente Cuesta C, González Fernández A, Cordero Torres G, Pérez López C, Peña Cortijo A, Conejero Hall L, Mateo Morales M, Rodríguez de la Peña A, Díez-Rivero CM, Rodríguez de Frías E, Guevara-Hoyer K, Fernández-Arquero M, Sánchez-Ramón S. Ochoa-Grullón J, et al. Front Immunol. 2021 Feb 12;11:611566. doi: 10.3389/fimmu.2020.611566. eCollection 2020. Front Immunol. 2021. PMID: 33679698 Free PMC article. - A Combination of Polybacterial MV140 and Candida albicans V132 as a Potential Novel Trained Immunity-Based Vaccine for Genitourinary Tract Infections.
Martin-Cruz L, Sevilla-Ortega C, Benito-Villalvilla C, Diez-Rivero CM, Sanchez-Ramón S, Subiza JL, Palomares O. Martin-Cruz L, et al. Front Immunol. 2021 Jan 21;11:612269. doi: 10.3389/fimmu.2020.612269. eCollection 2020. Front Immunol. 2021. PMID: 33552074 Free PMC article. - Mycobacterium tuberculosis PPE60 antigen drives Th1/Th17 responses via Toll-like receptor 2-dependent maturation of dendritic cells.
Su H, Zhang Z, Liu Z, Peng B, Kong C, Wang H, Zhang Z, Xu Y. Su H, et al. J Biol Chem. 2018 Jun 29;293(26):10287-10302. doi: 10.1074/jbc.RA118.001696. Epub 2018 May 8. J Biol Chem. 2018. PMID: 29739853 Free PMC article. - Sublingually administered bacterial lysates: rationale, mechanisms of action and clinical outcomes.
Braido F, Melioli G, Nicolini G, Ferraris M, Di Girolamo S, Di Gioacchino M, Canonica GW. Braido F, et al. Drugs Context. 2024 Aug 7;13:2024-1-5. doi: 10.7573/dic.2024-1-5. eCollection 2024. Drugs Context. 2024. PMID: 39165613 Free PMC article. Review.
Cited by
- Toward prevention of childhood ALL by early-life immune training.
Hauer J, Fischer U, Borkhardt A. Hauer J, et al. Blood. 2021 Oct 21;138(16):1412-1428. doi: 10.1182/blood.2020009895. Blood. 2021. PMID: 34010407 Free PMC article. - Combining different bacteria in vaccine formulations enhances the chance for antiviral cross-reactive immunity: a detailed in silico analysis for influenza A virus.
Bodas-Pinedo A, Lafuente EM, Pelaez-Prestel HF, Ras-Carmona A, Subiza JL, Reche PA. Bodas-Pinedo A, et al. Front Immunol. 2023 Aug 22;14:1235053. doi: 10.3389/fimmu.2023.1235053. eCollection 2023. Front Immunol. 2023. PMID: 37675108 Free PMC article. - MV140 mucosal bacterial vaccine improves uropathogenic E. coli clearance in an experimental model of urinary tract infection.
Saz-Leal P, Ligon MM, Diez-Rivero CM, García-Ayuso D, Mohanty S, Conejero L, Brauner A, Subiza JL, Mysorekar IU. Saz-Leal P, et al. Res Sq [Preprint]. 2023 Jun 7:rs.3.rs-2992611. doi: 10.21203/rs.3.rs-2992611/v1. Res Sq. 2023. PMID: 37333312 Free PMC article. Updated. Preprint. - Candida albicans V132 induces trained immunity and enhances the responses triggered by the polybacterial vaccine MV140 for genitourinary tract infections.
Martín-Cruz L, Angelina A, Baydemir I, Bulut Ö, Subiza JL, Netea MG, Domínguez-Andrés J, Palomares O. Martín-Cruz L, et al. Front Immunol. 2022 Nov 24;13:1066383. doi: 10.3389/fimmu.2022.1066383. eCollection 2022. Front Immunol. 2022. PMID: 36505433 Free PMC article. - Trained Immunity-Based Vaccines: A New Paradigm for the Development of Broad-Spectrum Anti-infectious Formulations.
Sánchez-Ramón S, Conejero L, Netea MG, Sancho D, Palomares Ó, Subiza JL. Sánchez-Ramón S, et al. Front Immunol. 2018 Dec 17;9:2936. doi: 10.3389/fimmu.2018.02936. eCollection 2018. Front Immunol. 2018. PMID: 30619296 Free PMC article. Review.
References
- Barnes, P. J. , Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy. Clin. Immunol. 2016. 138: 16–27. - PubMed
- Tejera‐Alhambra, M. , Palomares, O. , de Diego, R. P. , Diaz‐Lezcano, I. and Sanchez‐Ramon, S. , New biological insights in the immunomodulatory effects of mucosal polybacterial vaccines in clinical practice. Curr. Pharm. Des. 2016. 22: 6283–6293 - PubMed
- Sanchez‐Ramon, S. , de Diego, R. P. , Dieli‐Crimi, R. and Subiza, J. L. , Extending the clinical horizons of mucosal bacterial vaccines: current evidence and future prospects. Curr. Drug Targets 2014. 15: 1132–1143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous