Identification of CMTM6 and CMTM4 as PD-L1 protein regulators - PubMed (original) (raw)
. 2017 Sep 7;549(7670):106-110.
doi: 10.1038/nature23669. Epub 2017 Aug 16.
Chong Sun 1, Lucas T Jae 2, Raquel Gomez-Eerland 1, Evert de Vries 3, Wei Wu 4 5, Meike E W Logtenberg 1, Maarten Slagter 1 6, Elisa A Rozeman 1 7, Ingrid Hofland 8, Annegien Broeks 8, Hugo M Horlings 9, Lodewyk F A Wessels 6, Christian U Blank 1 7, Yanling Xiao 3, Albert J R Heck 4 5, Jannie Borst 3, Thijn R Brummelkamp 2 10 11, Ton N M Schumacher 1
Affiliations
- PMID: 28813410
- PMCID: PMC6333292
- DOI: 10.1038/nature23669
Identification of CMTM6 and CMTM4 as PD-L1 protein regulators
Riccardo Mezzadra et al. Nature. 2017.
Abstract
The clinical benefit for patients with diverse types of metastatic cancers that has been observed upon blockade of the interaction between PD-1 and PD-L1 has highlighted the importance of this inhibitory axis in the suppression of tumour-specific T-cell responses. Notwithstanding the key role of PD-L1 expression by cells within the tumour micro-environment, our understanding of the regulation of the PD-L1 protein is limited. Here we identify, using a haploid genetic screen, CMTM6, a type-3 transmembrane protein of previously unknown function, as a regulator of the PD-L1 protein. Interference with CMTM6 expression results in impaired PD-L1 protein expression in all human tumour cell types tested and in primary human dendritic cells. Furthermore, through both a haploid genetic modifier screen in CMTM6-deficient cells and genetic complementation experiments, we demonstrate that this function is shared by its closest family member, CMTM4, but not by any of the other CMTM members tested. Notably, CMTM6 increases the PD-L1 protein pool without affecting PD-L1 (also known as CD274) transcription levels. Rather, we demonstrate that CMTM6 is present at the cell surface, associates with the PD-L1 protein, reduces its ubiquitination and increases PD-L1 protein half-life. Consistent with its role in PD-L1 protein regulation, CMTM6 enhances the ability of PD-L1-expressing tumour cells to inhibit T cells. Collectively, our data reveal that PD-L1 relies on CMTM6/4 to efficiently carry out its inhibitory function, and suggest potential new avenues to block this pathway.
Conflict of interest statement
Competing financial interests
R.M., C.S., L.T.J., T.R.B. and T.N.M.S. are inventors on a patent application covering the use of CMTM6, CMTM4 and STUB1 as therapeutic and diagnostic targets. T.R.B. is co-founder and shareholder of Scenic Biotech. The other authors declare no competing interests.
Figures
Extended data Figure 1. PD-L1 is regulated by IFNγ and by the UTR in HAP1
(a) Flow cytometric analysis of PD-L1 expression of untreated HAP1 cells and HAP1 cells treated with the indicated concentrations of IFNγ. An IFNγ concentration of 0.5 ng/ml was chosen for the subsequent genetic screen, to allow identification of gene integrations that either enhance or suppress PD-L1 expression. Data are representative of three independent experiments. (b) Schematic representation of the PD-L1 gene and of the gene trap insertions observed in HAP1 cells sorted on the basis of either low or high PD-L1 expression. Note the bias towards integrations within introns 5 and 6 in the PD-L1 gene in PD-L1HI cells relative to PD-L1LOW cells, consistent with the structural variants beyond exon 4 of PD-L1 that have been shown to result in enhanced PD-L1 expression in a subset of adult T-cell leukaemia, diffuse large B-cell lymphoma, and stomach cancers. (c) Screen data as depicted in Fig. 1, but now with PD-L1 (CD274) data plotted when either including (CD274) or excluding (CD274*) integrations downstream of exon 5 (Refseq identifier NM_014143.3). MI, mutation index.
Extended data Figure 2. RNA expression of CMTM6 in human cancers and correlation with PD-L1 mRNA levels.
Pearson correlation coefficients are shown along with associated unadjusted p-values. As randomly selected genes are on average also weakly positively correlated (not shown), empirical p-values, which represent one minus the quantile of the CMTM6 and CD274 expression correlation coefficient among a reference distribution composed of correlation coefficients between CMTM6 and randomly selected genes, are also depicted. Empirical p-values smaller than .5 denote a stronger correlation between CMTM6 and CD274 than the median observed correlation in the reference distribution. TPM, transcript per million. ACC: adrenocortical carcinoma, BLCA: urothelial bladder carcinoma, BRCA: breast cancer, CESC: cervical squamous cell carcinoma, CHOL: cholangiocarcinoma, COAD: colorectal adenocarcinoma, DLBC: diffuse large B-cell lymphoma, ESCA: esophageal cancer, GBM: glioblastoma multiforme, HNSC: head and neck squamous, KICH: chromophobe renal cell carcinoma, KIRC: clear cell kidney carcinoma, KIRP: papillary kidney carcinoma, LAML: acute myeloid leukemia, LGG: lower grade glioma, LIHC: liver hepatocellular carcinoma, LUAD: lung adenocarcinoma, LUSC: lung squamous cell carcinoma, OV: ovarian serous cystadenocarcinoma, PAAD: pancreatic ductal adenocarcinoma, PCPG: pheochromocytoma and paraganglioma, PRAD: prostate adenocarcinoma, READ: rectum adenocarcinoma, SKCM: cutaneous melanoma, STAD: stomach cancer, TGCT: testicular germ cell cancer, THCA: papillary thyroid carcinoma, UCEC: uterine corpus endometrial carcinoma, UCS: uterine carcinosarcoma, UVM: uveal melanoma
Extended data Figure 3. Regulation of PD-L1 by CMTM6 in different tumor types.
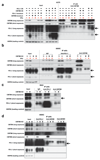
Flow cytometric analysis of PD-L1 expression of WM2664 melanoma**(a), COLO679 melanoma (c), DLD1 colorectal cancer (e), H2122 non-small lung cancer (o) cells and three short term cultures of melanoma xenografts (q), in which cells transduced independently with two vectors expressing different CMTM6 shRNAs are compared with cells transduced with control vector. Western blot analysis of CMTM6 and PD-L1 expression in WM2664 melanoma(b), COLO679 melanoma (d), DLD1 colorectal cancer (f), H2122 non-small lung cancer (p) cells, in which cells transduced independently with two vectors expressing different CMTM6 shRNAs are compared with cells transduced with control vector. Western blot analysis of CMTM6 and PD-L1 expression in WM2664 melanoma (b), COLO679 melanoma (d), DLD1 colorectal cancer (f) and H2122 non-small lung cancer(p)** cells in which cells transduced independently with two vectors expressing different CMTM6 shRNAs are compared with cells transduced with control vector. Flow cytometric analysis of PD-L1 expression as upon staining with anti-PD-L1 antibody or with PD-1-Fc in 8505c thyroid cancer**(g,h), RKO colorectal cancer (i,j) and H2030 non-small lung cancer (l,m) cells and western blot analysis of CMTM6 and PD-L1 expression in RKO colorectal cancer (k) and H2030 non-small cell lung cancer in which cells transduced independently with two vectors expressing different CMTM6 shRNAs are compared with cells transduced with control vector. In all cases, cells treated with IFNγ (25 ng/ml) or left untreated were compared. Panel g is identical to the one depicted in Fig. 2, shown again here to facilitate comparison. , Data are representative of three(c-n),** two (a,b,o,p) or one (q)independent experiments and were analyzed by unpaired t-test(a,c,e,g-m,o,q). Error bars represent s.d. of triplicates**(a,c,e,g-m,o,q)**. *P<0.05; **P<0.01; ***P<0.001. MFI, median fluorescence intensity; PDX, patient derived xenograft; NSCLC, non-small cell lung cancer; CRC, colorectal cancer.
Extended data Figure 4. CMTM4 and CMTM6, but not other CMTM family members, are regulators of PD-L1.
(a) Validation of CMTM6 and CMTM4 downregulation by Western blot analysis of cells shown in Fig. 3b. (b,c) Ectopic expression of CMTM4 restores IFNγ-induced PD-L1 expression in CMTM6-deficient cells. Two clones of CMTM6-deficient A375 cells (‘CMTM6 KO#6’ and ‘CMTM6 KO#12’) were transduced with retroviral vectors encoding CMTM4 (‘CMTM4 OE’) or CMTM6 (‘CMTM6 OE’) individually. After blasticidin selection, cells were cultured in the absence (‘untreated’) or presence of 25ng/ml IFNγ for 72 hours before analysis by flow cytometry (b), and Western blot analysis (c). Untransduced A375 parental cells served as controls. (d,e) Western blot analysis of expression of the indicated CMTM family members, as determined by staining with an anti-FLAG antibody. Two exposures of the same gel are shown. Expression of CMTM2 and 8 is not detected, and CMTM5 expression is low as compared to that of other CMTM family members. (f) Phylogenetic analysis of the CMTM family by CLUSTALW. CMTM6 and 4 form the two most closely related members. In view of the lack of detectable expression/ low expression observed for CMTM2, 8 and 5, an effect of these CMTM members on PD-L1 protein fate cannot be excluded. However, the observation that CMTM family members 7 and 3 that are more closely related to CMTM4 and 6 do not influence PD-L1 expression makes this unlikely. (g) Results of the flow cytometry based screen as shown in Fig. 1a, with the position of all CMTM family members indicated. Data are representative of two independent experiments (a-e). Error bars represent s.d. of triplicates (b). *P<0.05; **P<0.01; ***P<0.001. MFI, median fluorescence intensity; KO, knockout; OE, overexpression. MI, mutation index.
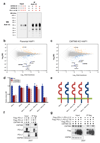
Extended data Figure 5. CMTM6 downregulation does not affect MHC class I and PD-L2 cell surface levels or PD-L1 mRNA levels and regulates PD-L1 stability after egress from the endoplasmic reticulum.
Flow cytometric analysis of MHC class I and PD-L2 expression in the panel of cell lines tested in Fig. 2 and Extended Data Fig. 3**(a), and in BM progenitor-derived DCs (b,c) in which cells transduced with control vector are compared with cells transduced independently with two vectors expressing different shRNA directed against CMTM6. For cell lines, cells treated with IFNγ (as indicated in the other figure legends) or left untreated were compared, for BM progenitor-derived DCs, cells treated with 500 ng/ml LPS or left untreated were compared. (d) qPCR analyses were performed to quantify relative mRNA levels of PD-L1 in the abovementioned tumor lines.(e)** Quantification of the experiment shown in Fig. 4c**(f)** Immunoprecipitates of the same samples as used in Fig. 4c were either mock treated, treated with EndoH, or treated with PNGaseF to examine the kinetics of protein maturation. No relevant difference in maturation kinetics were observed between cells overexpressing CMTM6 and CMTM6-deficient cells. Pulse chase experiments were performed three times, once comparing CMTM6 overexpressing and CMTM6-deficient cells (a), and twice comparing wt and CMTM6-deficient cells. Other data are representative of at least two independent experiments. MFI, median fluorescence intensity; BM, bone marrow; DC, dendritic cell; KO, knockout; OE, overexpression; EndoH, endoglycosidase H; PNGaseF, peptide-N-glycosidase F..
Extended data Figure 6. Specificity and membrane localization of CMTM6, and co-localization with PD-L1 in human tumors.
(a), Membrane-fractionated proteome of 8505C and RKO cells. CMTM6 and PD-L1 were detected by LC-MS/MS predominantly from the plasma membrane fractions. Label-free quantification (LFQ) performed by intensity-based normalization of 4 fractions together across different cell lines is depicted. (b) A375 parental cells, CMTM6 KO or CMTM6 overexpressing cells were fixed and formalin embedded, and stained for CMTM6 with a monoclonal antibody (1D6) generated against a peptide from the C-terminal domain of CMTM6. Analysis shows mainly membranous stain, as indicated by the arrowheads. (c) Sequential slides from lymph node and subcutaneous metastases from 3 melanoma patients were stained for PD-L1 (left) or for CMTM6 (right), showing frequent localization of PD-L1 within CMTM6 positive areas. In total, samples from 9 melanoma patients and 5 PD-L1 positive lung cancer samples were analyzed. OE, overexpression; KO, knockout.
Extended data Figure 7. Interactions between CMTM6, PD-L1, and CMTM4, and effect of CMTM6 on PD-L1 stability.
(a) A375 parental cells, CMTM6-deficient cells, PD-L1-deficient cells, and cells ectopically expressing CMTM6 or PD-L1, were cultured in the absence or presence of 25ng/ml IFNγ for 48 hours before preparation of cell lysates. Immunoprecipitation was performed using a CMTM6-specific antibody immobilized on protein A coated beads. Immunoprecipitates and whole cell lysate were subjected to SDS-PAGE and immunoblotted for CMTM6 and PD-L1. Two exposures of the same western blot are shown. Arrows indicate PD-L1 bands. (b) parental 8505C cells and CMTM6-deficient 8505C cells were cultured in the absence or presence of 50ng/ml IFNγ for 72 hours before preparation of cell lysates. Immunoprecipitation was performed using CMTM6- or PD-L1-specific antibodies immobilized on protein A coated beads. Immunoprecipitates and whole cell lysates were subjected to SDS-PAGE and immunoblotted for CMTM6 and PD-L1. Two exposures of the same western blot are shown. Normal IgG served as control. Arrows indicate PD-L1 bands. (c, d) Parental and CMTM6 knockout RKO cells (c) and (d) 8505c cells were lysed and immunoprecipitation was performed using antibodies immobilized on protein G coated beads as indicated. Immunoprecipitates and whole cell lysates were subjected to SDS-PAGE, and Western blot analysis of CMTM4 and PD-L1 was carried out. Two exposures of the same Western blots are shown. Arrows indicate CMTM4. Data are representative of three independent experiments. KO, knockout; OE, overexpression.
Extended data Figure 8. Aspects of PD-L1 regulation by CMTM6 and STUB1.
(a) V5-tagged PD-L1 was introduced into parental, CMTM6-overexpressing and CMTM6-deficient A375 cells. Cell lysates were denatured and then subjected to immunoprecipitation with anti-V5 antibody immobilized on protein G-coated beads. Immunoprecipitates were then analyzed by immunoblotting with anti-V5 antibody as a control for the experiments shown in Fig. 4e. Results of the FACS-based genetic screens in CMTM6 expressing and CMTM6 deficient HAP1 cells as shown in Fig. 1a**(b)** and in Fig. 3a**(c), with the position of STUB1 indicated. (d)Relative expression of PD-L1, PD-L2 and the indicated PD-L1 – PD-L2 chimeric proteins in CMTM6 KD A375 cells as compared to matched control. Chimeras were detected with an anti PD-L1 or an anti PD-L2 antibody.(e) Schematic overview of the chimeric proteins analyzed.(f,g)** 293T human embryonic kidney cells were co-transfected with a vector encoding either PD-L1, PD-L2 or the indicated chimeric protein, together with a vector encoding CMTM6. Cell lysates were denatured and subjected to immunoprecipitation with anti-flag antibody immobilized on protein G-coated beads. Lysates and immunoprecipitates were then analyzed by immunoblotting with the indicated antibodies. Data are representative of three (a,d), one (f) or two **(g)**independent experiments. Error bars represent s.d. of triplicates. MFI, median fluorescence intensity; KO, knockout; OE, overexpression; TM, transmembrane; IC, intracellular; EC, extracellular.
Extended data Figure 9. Orientation mapping of CMTM6.
(a) Predicted domain topology of CMTM6 according to TMHMM Server v. 2.0 (
http://www.cbs.dtu.dk/services/TMHMM/
). **(b,c)**A375 cells were transduced with C- or N-terminal HA epitope tagged CMTM6. HA staining was performed in both live cells and fixed and permeabilized cells followed by flow cytometry analysis and quantified in (c). MFI, median fluorescence intensity.
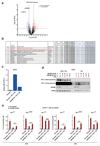
Extended data Figure 10. Selectivity of CMTM6 and CMTM6 loss alleviates PD-L1-mediated T cell suppression.
(a) Comparative membrane-fractionated mass spectrometry of CMTM6 proficient or deficient RKO cells. 4 wild type and 4 CMTM6 KO RKO clones were analyzed by LC-MS/MS and differential protein abundance is shown in a volcano plot. (b) Table indicating proteins found up- or down-regulated upon CMTM6 removal in both 8505c and RKO. Flow cytometric**(c)** and Western blot (d) analysis of CMTM6 and PD-L1 expression in parental A375 or CMTM6 deficient A375 clones in which PD-L1 is ectopically expressed by lentiviral transduction. (e). Primary human T cells were transduced with the MART-1 specific 1D3 TCR and PD-1. Transduced T cells were co-cultured with unloaded or MART-1 peptide loaded PD-L1-overexpressing A375 cells (‘Parental + PD-L1 OE’), parental A375 cells (‘Parental’), or CMTM6-deficient A375 cells that overexpressed PD-L1 (‘CMTM6 KO+PD-L1 OE’). IL-2 production in T cells that expressed high, intermediate, or low levels of PD-1 (‘PD-1HI’, ‘PD-1INTER’ or ‘PD-1LOW’) were analyzed by flow cytometry. Untransduced A375 cells (‘Parental’) served as controls. Data are representative of three independent experiments and were analyzed by unpaired t-test (c). Error bars represent s.d. of triplicates. *P<0.05; **P<0.01; ***P<0.001. KO, knockout; OE, overexpression; TM, transmembrane; PM, plasma membrane.
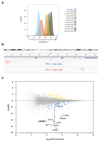
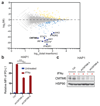
Figure 1. Identification of CMTM6 as a modulator of PD-L1 expression.
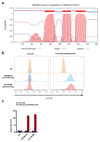
(a) Flow cytometry-based screen for modulators of PD-L1 cell surface expression in HAP1 cells. Dots represent individual genes, X axis indicates the number of disruptive insertions per gene, Y axis the frequency of independent insertions in the PD-L1HI channel over the frequency of insertions in the PD-L1LOW channel for each gene. Light blue and orange dots indicate genes with significant enrichment of insertions (FDR-corrected P-value, FCPv<10-6) within the PD-L1LOW and PD-L1HI population, respectively. Dark blue circles indicate known components of the IFNγR signaling pathway plus IRF1 and CMTM6 (in bold). The purple dot represents PD-L1 (CD274*) when excluding integrations downstream of exon 5 (Refseq identifier NM_014143.3). See
for interactive graphs.(b) Relative PD-L1 cell surface expression in control or independent CMTM6 knockdown HAP1 cells, either with or without IFNγ exposure. (c) Validation of CMTM6 knockdown by Western Blot. Data are representative of one (a) or at least three **(b,c)**independent experiments, and were analyzed by unpaired t-test (b). Error bars represent s.d. of triplicates (b). *P<0.05; **P<0.01; ***P<0.001. MFI, median fluorescence intensity; MI, mutation index.
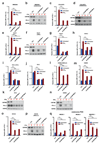
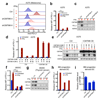
Figure 2. CMTM6 regulates PD-L1 expression in different tumor types and primary dendritic cells.
(a,b,f) Relative PD-L1 cell surface expression in control or independent CMTM6 knockdown A375 melanoma cells (a-b), 8505c thyroid cancer cells (f), either with or without IFNγ exposure. (c,g) Western blot analysis of CMTM6 and PD-L1 expression in control or independent CMTM6 knockdown A375 melanoma cells (c)or 8505c thyroid cancer cells (g), either with or without IFNγ exposure. Each cell line was tested in at least three independent experiments, representative results are shown. (d) Flow cytometric analysis, and (e) western blot analysis of PD-L1 expression in parental, CMTM6 deficient, CMTM6 overexpressing and CMTM6 reconstituted A375 melanoma cells. (l) Flow cytometric analysis of PD-L1 expression in control or independent CMTM6 knockdown primary BM progenitor-derived DCs, either with or without LPS exposure. (m) Knockdown efficiency of CMTM6 in primary BM progenitor-derived DCs. Data are representative of at least three(a-k) or two (l, m) independent experiments and were analyzed by unpaired t-test (b,f,h,j). Error bars represent s.d. of triplicates (b,d,f,h,j,m). *P<0.05; **P<0.01; ***P<0.001. MFI, median fluorescence intensity; BM, bone marrow; DC, dendritic cell.
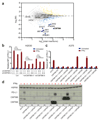
Figure 3. Identification of CMTM4 as a second PD-L1 regulator.
(a) Haploid genetic screen for modulators of PD-L1 cell surface expression in CMTM6-deficient HAP1 cells. (b) PD-L1 surface expression of parental, CMTM6 knockdown, CMTM4 knockdown, or double knockdown H2030 cells, either with or without IFNγ exposure. (c) Flow cytometric analysis and (d) western blot analysis of PD-L1 expression in parental A375, CMTM6 KO A375, and CMTM6 KO A375 reconstituted with the indicated CMTM family member, either with or without IFNγ exposure. Data are representative of one (a), two (b) or three**(c,d)** independent experiments and were analyzed by unpaired t-test (b). Error bars represent s.d. of triplicates**(b)**. *P<0.05; **P<0.01; ***P<0.001. MFI, median fluorescence intensity; KO, knockout. MI, mutation index.
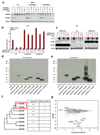
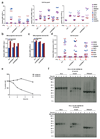
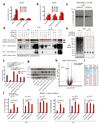
Figure 4. CMTM6 forms a molecular partner of PD-L1 and regulates PD-L1 protein stability.
Time course of PD-L1 surface protein (a) or mRNA (b)levels in A375 cells upon IFNγ exposure (c) SDS-PAGE analysis of anti-V5 immunoprecipitates from V5-PD-L1 overexpressing CMTM6 KO or CMTM6 overexpressing A375 cells at different time points after 35S methionine/cysteine labeling. (d) Immunoblots of lysates or anti-PD-L1 immunoprecipitates from the indicated A375 melanoma cells, either with or without IFNγ exposure Arrows indicate CMTM6. (e)Immunoblots of lysates or anti-V5 immunoprecipitates from V5-PD-L1 overexpressing parental, CMTM6 KO or CMTM6 overexpressing A375 cells. PD-L1 surface protein (f) or lysate immunoblots (g) of parental, CMTM6 KO, STUB1 KO or double KO A375 cells. (h)Comparative membrane-fractionated mass spectrometry of 4 CMTM6 proficient and 4 CMTM6 deficient 8505c clones. (i) Overview of proteins consistently found up- or down-regulated upon CMTM6 removal in both 8505c and RKO.(j) IL-2 production by PD-1HI, PD-1INTERand PD-1NEG primary human T cells obtained by transduction with the MART-1 specific 1D3 TCR and PD-1, and co-cultured with unloaded or MART-I peptide-loaded parental or CMTM6 KO 8505C cells. Data are representative of two(a, b), one (c,h) or three(d,e,f,g,j) independent experiments and were analyzed by unpaired t-test (f,j). Error bars represent s.d. of triplicates**(a,b,f,j)**. *P<0.05; **P<0.01; ***P<0.001. MFI, median fluorescence intensity; KO, knockout; OE, overexpression.
Comment in
- Novel regulators of PD-L1 expression in cancer: CMTM6 and CMTM4-a new avenue to enhance the therapeutic benefits of immune checkpoint inhibitors.
Imamovic D, Vranic S. Imamovic D, et al. Ann Transl Med. 2017 Dec;5(23):467. doi: 10.21037/atm.2017.09.32. Ann Transl Med. 2017. PMID: 29285500 Free PMC article. No abstract available. - CMTM6 stabilizes PD-L1 expression and refines its prognostic value in tumors.
Mamessier E, Birnbaum DJ, Finetti P, Birnbaum D, Bertucci F. Mamessier E, et al. Ann Transl Med. 2018 Feb;6(3):54. doi: 10.21037/atm.2017.11.26. Ann Transl Med. 2018. PMID: 29610746 Free PMC article. No abstract available.
Similar articles
- Expression Analysis of Canine CMTM6 and CMTM4 as Potential Regulators of the PD-L1 Protein in Canine Cancers.
Takeuchi H, Konnai S, Maekawa N, Minato E, Ichikawa Y, Kobayashi A, Okagawa T, Murata S, Ohashi K. Takeuchi H, et al. Front Vet Sci. 2020 Jun 11;7:330. doi: 10.3389/fvets.2020.00330. eCollection 2020. Front Vet Sci. 2020. PMID: 32596272 Free PMC article. - CMTM6 and CMTM4 as two novel regulators of PD-L1 modulate the tumor microenvironment.
Zhang T, Yu H, Dai X, Zhang X. Zhang T, et al. Front Immunol. 2022 Jul 25;13:971428. doi: 10.3389/fimmu.2022.971428. eCollection 2022. Front Immunol. 2022. PMID: 35958549 Free PMC article. Review. - CMTM6, a potential immunotherapy target.
Liang J, Li S, Li W, Rao W, Xu S, Meng H, Zhu F, Zhai D, Cui M, Xu D, Cai J, Zhang B. Liang J, et al. J Cancer Res Clin Oncol. 2022 Jan;148(1):47-56. doi: 10.1007/s00432-021-03835-9. Epub 2021 Nov 16. J Cancer Res Clin Oncol. 2022. PMID: 34783871 Review. - CMTM6 as a master regulator of PD-L1.
Yaseen MM, Abuharfeil NM, Darmani H. Yaseen MM, et al. Cancer Immunol Immunother. 2022 Oct;71(10):2325-2340. doi: 10.1007/s00262-022-03171-y. Epub 2022 Mar 16. Cancer Immunol Immunother. 2022. PMID: 35294592 Free PMC article. Review. - The clinical and prognostic significance of CMTM6/PD-L1 in oncology.
Yaseen MM, Abuharfeil NM, Darmani H. Yaseen MM, et al. Clin Transl Oncol. 2022 Aug;24(8):1478-1491. doi: 10.1007/s12094-022-02811-0. Epub 2022 Mar 12. Clin Transl Oncol. 2022. PMID: 35278198 Review.
Cited by
- An Update on the Role of Ubiquitination in Melanoma Development and Therapies.
Soysouvanh F, Giuliano S, Habel N, El-Hachem N, Pisibon C, Bertolotto C, Ballotti R. Soysouvanh F, et al. J Clin Med. 2021 Mar 8;10(5):1133. doi: 10.3390/jcm10051133. J Clin Med. 2021. PMID: 33800394 Free PMC article. Review. - MPscore: A Novel Predictive and Prognostic Scoring for Progressive Meningioma.
Liu F, Qian J, Ma C. Liu F, et al. Cancers (Basel). 2021 Mar 5;13(5):1113. doi: 10.3390/cancers13051113. Cancers (Basel). 2021. PMID: 33807688 Free PMC article. - Expression Analysis of Canine CMTM6 and CMTM4 as Potential Regulators of the PD-L1 Protein in Canine Cancers.
Takeuchi H, Konnai S, Maekawa N, Minato E, Ichikawa Y, Kobayashi A, Okagawa T, Murata S, Ohashi K. Takeuchi H, et al. Front Vet Sci. 2020 Jun 11;7:330. doi: 10.3389/fvets.2020.00330. eCollection 2020. Front Vet Sci. 2020. PMID: 32596272 Free PMC article. - Ubiquitin-specific peptidase 10, a deubiquitinating enzyme: Assessing its role in tumor prognosis and immune response.
Ye Z, Chen J, Huang P, Xuan Z, Zheng S. Ye Z, et al. Front Oncol. 2022 Sep 28;12:990195. doi: 10.3389/fonc.2022.990195. eCollection 2022. Front Oncol. 2022. PMID: 36248971 Free PMC article. Review. - Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, _BRCA1_-like status, tumor-infiltrating immune cells and survival.
Sobral-Leite M, Van de Vijver K, Michaut M, van der Linden R, Hooijer GKJ, Horlings HM, Severson TM, Mulligan AM, Weerasooriya N, Sanders J, Glas AM, Wehkamp D, Mittempergher L, Kersten K, Cimino-Mathews A, Peters D, Hooijberg E, Broeks A, van de Vijver MJ, Bernards R, Andrulis IL, Kok M, de Visser KE, Schmidt MK. Sobral-Leite M, et al. Oncoimmunology. 2018 Sep 11;7(12):e1509820. doi: 10.1080/2162402X.2018.1509820. eCollection 2018. Oncoimmunology. 2018. PMID: 30524905 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials