CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity - PubMed (original) (raw)
. 2017 Sep 7;549(7670):101-105.
doi: 10.1038/nature23643. Epub 2017 Aug 16.
Marian L Burr 1 2 3, Yih-Chih Chan 1, James C Williamson 3, Katherine Woods 4 5, Paul A Beavis 1 2, Enid Y N Lam 1 2, Melissa A Henderson 1 2, Charles C Bell 1 2, Sabine Stolzenburg 1, Omer Gilan 1 2, Stuart Bloor 3, Tahereh Noori 1, David W Morgens 6, Michael C Bassik 6, Paul J Neeson 1 2, Andreas Behren 4 5, Phillip K Darcy 1 2, Sarah-Jane Dawson 1 2 7, Ilia Voskoboinik 1 2, Joseph A Trapani 1 2, Jonathan Cebon 4 5, Paul J Lehner 3, Mark A Dawson 1 2 7 8
Affiliations
- PMID: 28813417
- PMCID: PMC5706633
- DOI: 10.1038/nature23643
CMTM6 maintains the expression of PD-L1 and regulates anti-tumour immunity
Marian L Burr et al. Nature. 2017.
Abstract
Cancer cells exploit the expression of the programmed death-1 (PD-1) ligand 1 (PD-L1) to subvert T-cell-mediated immunosurveillance. The success of therapies that disrupt PD-L1-mediated tumour tolerance has highlighted the need to understand the molecular regulation of PD-L1 expression. Here we identify the uncharacterized protein CMTM6 as a critical regulator of PD-L1 in a broad range of cancer cells, by using a genome-wide CRISPR-Cas9 screen. CMTM6 is a ubiquitously expressed protein that binds PD-L1 and maintains its cell surface expression. CMTM6 is not required for PD-L1 maturation but co-localizes with PD-L1 at the plasma membrane and in recycling endosomes, where it prevents PD-L1 from being targeted for lysosome-mediated degradation. Using a quantitative approach to profile the entire plasma membrane proteome, we find that CMTM6 displays specificity for PD-L1. Notably, CMTM6 depletion decreases PD-L1 without compromising cell surface expression of MHC class I. CMTM6 depletion, via the reduction of PD-L1, significantly alleviates the suppression of tumour-specific T cell activity in vitro and in vivo. These findings provide insights into the biology of PD-L1 regulation, identify a previously unrecognized master regulator of this critical immune checkpoint and highlight a potential therapeutic target to overcome immune evasion by tumour cells.
Conflict of interest statement
The authors have no competing interests to declare.
Figures
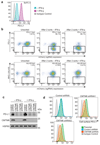
Extended Data Figure 1. A CRISPR/Cas9 screen in the pancreatic cancer cell line BxPC-3 identifies CMTM6 as a principal regulator of PD-L1 expression
a. PD-L1 is expressed at the cell surface in BxPC-3 cells and upregulated following treatment with IFN-γ. Cell surface PD-L1 was analysed by flow cytometry following incubation with or without IFN-γ 500IU/ml for 48 h. b. BxPC-3 cells stably expressing Cas9 were mutagenised by infection with a pooled lentiviral sgRNA library and rare PD-L1 low cells enriched by two successive rounds of FACS sorting, with or without IFN-γ pretreatment as indicated, for mCherry positive (containing sgRNA vector) PD-L1 low cells. FACS plots depict sorted and unsorted mutagenised populations stained for PD-L1 (top panel) and MHC class I control (bottom panel). Cells were pre-treated with IFN-γ 500IU/ml for 48 h prior to analysis. c. Total cellular PD-L1 levels are dramatically reduced following shRNA-mediated depletion of CMTM6 in both IFN-γ treated and non-IFN-γ treated cells. Immunoblot for PD-L1, CMTM6 and HSP60 control in MDA-MB-231 cells transduced with shRNAs targeting CMTM6 or control shRNA. d. Reduced cell surface PD-L1 following shRNA-mediated depletion of CMTM6 in BxPC-3 cells.
Extended Data Figure 2. Down-regulation of cell surface PD-L1 following effective RNA interference (RNAi)-mediated depletion of CMTM6.
a. Efficiency of CMTM6 depletion was assessed by quantitative reverse transcription PCR (qRT-PCR) in cells transduced with shRNAs targeting CMTM6 or a non-targeting control shRNA. The mean and s.e.m. of 3 technical replicates are shown. b. RNAi-mediated depletion of CMTM6 reduces cell surface PD-L1 expression both in the presence and absence of IFN-γ stimulation. Cell surface PD-L1 was analysed by flow cytometry in the indicated cell lines transduced with lentiviral vectors encoding shRNAs targeting CMTM6 or control shRNA (left panel). The adjacent bar chart (right panel) shows fold change in cell surface PD-L1 fluorescence intensity following CMTM6 depletion (mean and s.e.m.). BxPC-3, HCC-827 and A375 cells were pre-treated with IFN-γ 500IU/ml for 48 h prior to analysis. Experiments were performed at least twice in each cell type.
Extended Data Figure 3. CRISPR/Cas9-mediated knockout of CMTM6 reduces cell surface PD-L1 expression in a broad range of cancer cells.
Flow cytometry analysis of cell surface PD-L1 in cells transduced with different sgRNAs targeting CMTM6 compared to parental Cas9 expressing cells. Cell lines depicted are melanoma (A375 and WM-852), triple-negative breast cancer (MDA-MB-231), non-small cell lung cancer (HCC-827) and pancreatic cancer (BxPC-3). All cells were pre-treated with IFN-γ 500IU/ml for 48 h prior to analysis. Selected representative histograms are presented in Figure 1e.
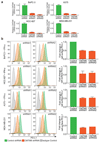
Extended Data Figure 4. CMTM6 selectively regulates both constitutive and interferon-induced PD-L1 without compromising tumour cell MHC class I expression.
a. Cell surface MHC class I expression is unaffected by shRNA-mediated depletion of CMTM6. Flow cytometry analysis of surface MHC class I and PD-L1 in MDA-MB-231 and A375 cells transduced with shRNAs targeting CMTM6 or control shRNA. Cells were pre-treated with IFN-γ 500IU/ml for 48 h prior to analysis. b. CMTM6 knockout leads to loss of both constitutive and IFN-γ-induced PD-L1 expression. Flow cytometry analysis of cell surface PD-L1 in Cas9-expressing MDA-MB-231 cells transduced with sgRNA targeting either PD-L1, JAK2 or CMTM6 (red) compared to parental Cas9-expressing cells (blue). PD-L1 expression is reduced in both the presence and absence of IFN-γ stimulation following knockout of CMTM6. In contrast, knockout of the IFN-γ pathway component JAK2 leads to selective loss of IFN-γ induced upregulation of PD-L1 with no effect on constitutive PD-L1 expression.
Extended Data Figure 5. CMTM6 is not regulated by IFN-γ and co-localises with PD-L1 at the cell surface in both the presence and absence of IFN-γ stimulation.
a. CMTM6 regulates cell surface and total PD-L1 levels in a dose-dependent manner. Single cell clones isolated from WM-852 cells transduced with Cas9 and an sgRNA targeting CMTM6 were analysed by flow cytometry for surface PD-L1 and immunoblot for total PD-L1 and CMTM6 following incubation with 500IU/ml IFN-γ for 48 h b. qRT-PCR analysis of relative CMTM6 mRNA expression in the indicated cell lines incubated with or without IFN-γ 500IU/ml for 48 h prior to analysis. Graphs show mean and s.e.m. of two biological replicates (MDA-MB-231 and HCC-827) or three technical replicates (BxPC-3). c. To confirm specific staining of endogenous CMTM6 by the anti-CMTM6 antibody, CMTM6 knockout MDA-MB-231 cells were labelled with CFSE prior to mixing with wildtype MDA-MB-231 cells, fixed and immunostained for CMTM6. Scale bar: 10μm. d. MDA-MB-231 cells treated with IFN-γ 500IU/ml for 48 h were fixed, immunostained for CMTM6 and PD-L1 and analysed by confocal microscopy. Scale bar: 10μm.
Extended Data Figure 6. CMTM6 shows functional specificity for PD-L1
a&b. CMTM6 specifically regulates PD-L1, with no effect on cell surface PD-L2 levels in THP-1 cells. THP-1 cells expressing Cas9 and transduced with CMTM6 sgRNA were treated with IFN-γ 500 IU ml-1 and TNF-α 20 ng ml-1 for 24 h as indicated prior to staining of cell surface PD-L1 and PD-L2 and analysis by flow cytometry. C. Mass spectrometry analysis identifies PD-L1 as a principal interaction partner of CMTM6. CMTM6 was immunoprecipitated from digitonin lysates of IFN-γ-treated MDA-MB-231 cells, and CMTM6 knockout cells as a control. Immunoprecipitates were analysed by mass spectrometry. Table represents all CMTM6 interaction partners identified by at least 3 unique peptides and absent from the control immunoprecipitation from CMTM6 knockout cells. d. Increased degradation of EndoH-resistant PD-L1 species in the absence of CMTM6. IFN-γ treated CMTM6 knockout or parental Cas9-expressing WM-852 cells were pulse-labelled with 35S-methionine/cysteine for 30 min before chase incubation at 37°C. PD-L1 was immunoprecipitated from detergent lysates at the indicated times. e&f. IFN-γ 500IU/ml was added to CMTM6 knockout and parental Cas9-expressing WM-852 cells and samples taken at the indicated time-points for analysis by flow cytometry. A representative example of an experiment done in biological triplicate is shown. g. Lysates of CMTM6 knockout cells incubated with IFN-γ for the indicated times were treated with or without EndoH prior to SDS-PAGE analysis and immunoblot to assess modification of N-linked glycans on PD-L1 as a marker of passage through the Golgi.
Extended Data Figure 7. Cellular Localisation of PD-L1
a) Loss of CMTM6 leads to selective destabilisation of PD-L1 with no effect on the stability of cell surface MHC class I. IFN-γ treated CMTM6 knockout and Cas9 control WM-852 cells were labelled as described in Figure 3B with APC-conjugated antibodies specific for either PD-L1 or MHC class I on ice prior to incubation at 37°C. Samples taken at the indicated time-points were analysed by flow cytometry. Experiment performed twice. b) MDA-MB-231 cells were fixed, immunostained for PD-L1 together with a marker of the ER (CANX), Golgi (GM130 and TGN46), early endosome (EEA1) or late endosome/lysosome (LAMP1) prior to analysis by confocal microscopy. Scale bar: 10μm.
Extended Data Figure 8. PD-L1 endocytosis and recycling assays
a. Internalisation assay (relates to data presented in Figure 3g). Cells were labelled with unconjugated PD-L1-specific antibodies at 4°C. After washing off unbound antibody, cells were incubated at 37°C to allow PD-L1 internalisation. Remaining cell surface antibody-labelled PD-L1 was detected with an AF647-conjugated anti-mouse secondary antibody and analysed by flow cytometry. b. Recycling assay 1. Cell surface PD-L1 in IFN-γ treated CMTM6 knockout and Cas9 control WM-852 cells was labelled with an unconjugated PD-L1 specific antibody and allowed to internalise for 30 min at 37°C as described in a. Remaining cell-surface bound antibody was stripped by washing in pH 2.5 buffer and cells were either kept on ice (post-strip baseline) or re-incubated at 37°C for the indicated times. Recycled PD-L1 was detected with an AF647-conjugated anti-mouse secondary antibody. c. Recycling assay 2 (relates to data presented in Figure 3h). Cells were labelled with an AF488-conjugated PD-L1 specific antibody, washed and incubated at 37°C to allow endocytosis. Remaining cell surface AF488 was quenched by incubation with an AF488-specific antibody for 1 h on ice prior to reincubation at 37°C to allow recycling of antibody-labelled PD-L1. Samples were split and incubated with or without an AF488-specific antibody for 1 h on ice to re-quench cell surface AF488. Recycled PD-L1 was detected by the reappearance of cell surface ‘quenchable’ AF488 signal. After normalisation for incomplete quenching at baseline by subtracting the ‘quenchable’ AF488 fluorescence at time zero, the fluorescence intensity of recycled PD-L1 at each time-point was compared to that of the total internalised PD-L1 after the endocytosis step to calculate the proportion of PD-L1 recycled.
Extended Data Figure 9. PD-L1 is targeted for degradation in the lysosome following depletion of CMTM6
a/b. PD-L1 is targeted for lysosome-dependent degradation in the absence of CMTM6. IFN-γ treated CMTM6 knockout and Cas9 control WM-852 cells were labelled with APC-conjugated PD-L1-specific antibody as in Figure 3b. Cells were chased at 37°C in the presence or absence of either 50nM Concanamycin-A, 50μM Chloroquine or 400nM Bafilomycin A1. 2 experiments (mean, s.e.m.) c. IFN-γ treated CMTM6 knockout and Cas9 control WM-852 cells were incubated with Con-A, Chloroquine or Bafilomycin-A1 for 16 h prior to analysis by immunoblot. d. Following inhibition of lysosomal degradation PD-L1 remains sequestered intracellularly in the absence of CMTM6. MDA-MB-231 cells expressing shRNAs targeting CMTM6 or control shRNA were incubated with 50nM Concanamycin-A (ConA) for 16 h prior to flow cytometry analysis for cell surface PD-L1. e. Jurkat cells co-cultured with a CMTM6 knockout tumour show enhanced Il-2 secretion. Jurkat cells were pre-activated overnight with PMA and PHA and co-cultured with PD-L1 knockout, CMTM6 knockout or control vector expressing WM-852 Cas9 cells pre-treated with 500IU/ml IFN-γ for 24h to upregulate PD-L1 expression. Il-2 levels in the culture supernatant were measured by cytometric bead array after 48 h and 72 h co-culture of Jurkat cells and tumour. Graphs show percent change in Il-2 secretion compared to control cell co-culture (mean and s.e.m. of experimental duplicates) f-h. Reduction in cell surface PD-L1 following knockout or knockdown of CMTM6 in NY-ESO-1 expressing melanoma cell lines used in T-cell co-culture assays. All cells were treated with IFN-γ prior to flow cytometry analysis for cell surface PD-L1. f. A375 cells stably expressing Cas9 were transduced with two different sgRNA targeting CMTM6 and CMTM6 knockout clones isolated by dilution cloning. g. Patient-derived melanoma cell line LM-MEL-44 was transduced with CMTM6 targeting or control shRNA. h. Patient-derived melanoma cell line LM-MEL-53 was sequentially transduced with Cas9 and either one of two different sgRNAs targeting CMTM6, an sgRNA targeting PD-L1 or a control vector.
Extended Data Figure 10. CMTM6 regulates tumour-specific T cell activity by regulating PD-L1 levels
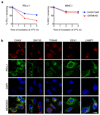
a. Schematic overview of T-cell and tumour co-culture assays to evaluate PD-L1-dependent inhibition of T-cell activity. b. Increased PD-1 expression on NY-ESO-192-100 HLA-CW3 restricted T-lymphocytes following 24 h co-culture with LM-MEL-53 melanoma. Staining with APC-conjugated anti-PD-1 antibody is completely blocked following addition of anti-PD-1 antibody nivolumab (BMS) at a saturating concentration (10μg/ml). c-fg CMTM6 regulates the anti-tumour activity of NY-ESO-1 antigen-specific T lymphocytes c-e. NY-ESO-192-100 HLA-CW3 restricted or NY-ESO-1157-165 HLA-A2 restricted CTLs were incubated with the indicated melanoma cells at an effector:target ratio of 1:1 (c&e) or 1:2 (d) for 24-48 h at 37 °C, then washed off. CTL mediated tumour lysis was determined by MTS assay and normalized to control wells with no CTLs.. Error bars represent SEM (n = 3). * p < 0.05. f&g. Enhanced T-cell activation following co-culture with a CMTM6-depleted cancer cells. f. NY-ESO-192-100 HLA-CW3 restricted T-lymphocytes were cultured with CMTM6 sgRNA, PD-L1 sgRNA or control vector transduced LM-MEL-53 melanoma cells stably expressing Cas9 for 24h prior to incubation with Brefeldin A for 6 h and intracellular staining for TNF-α. Where indicated, 10μg/ml nivolumab (anti-PD-1 antibody) was added to the co-cultures. c-f. Graphs show triplicates (mean + s.e.m.) *P < 0.05, unpaired two-tailed t-test. g. Supernatants from 3 day co-cultures of NY-ESO-192-100 CTLs and control vector or CMTM6 sgRNA transduced LM-MEL-53 cells were analysed by multiplex bead immunoassay (Luminex). Chemokines detected at increased levels following inclusion of 10μg/ml nivolumab (anti-PD-1 antibody) in the co-cultures are shown. Graphs show triplicates (mean + s.e.m). * p < 0.05, unpaired two-tailed t-test with Holm-Sidak correction for multiple testing. h. Efficiency of CMTM6 depletion was assessed by qRT-PCR in B16-OVA cells transduced with shRNA targeting CMTM6 or a non-targeting control shRNA. i&j. Growth of CMTM6-deficient and control shRNA expressing B16-OVA tumours injected subcutaneously in C57BL/6 mice. Data are from two independent experiments. Where indicated, mice were treated with anti-CTLA-4 or isotype control antibody from day 4 after tumour cell injection.
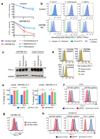
Figure 1. CMTM6 is a principal regulator of PD-L1 expression in multiple tumour types
a. A genome-wide CRISPR/Cas9 screen identifies genes essential for cell surface PD-L1 expression. BxPC-3 pancreatic cancer cells expressing Cas9 were mutagenised with a pooled lentiviral sgRNA library and PD-L1 low cells enriched by FACs sorting b&c. Significant hits from screens in cells pre-treated with IFN-γ before sorting (B) and non-IFN-γ treated cells (C). Dotted line indicates Bonferroni-corrected significance threshold. d. Immunoblot in MDA-MB-231 cells expressing Cas9 and sgRNAs targeting either CMTM6 or PD-L1. e. Surface PD-L1 in IFN-γ-treated cells transduced with CMTM6-specific sgRNAs versus parental Cas9 expressing cells. See Extended Data Fig. 3 for full dataset. f. PD-L1 expression in CMTM6 knockout MDA-MB-231 cells ± CMTM6 cDNA analysed by flow cytometry and immunoblot. Representative of 3 experiments. g. qRT-PCR analysis in control and CMTM6-depleted cells treated ± 500IU/ml IFN-γ for 48h. 2 biological replicates (mean, s.e.m.).
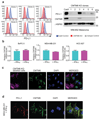
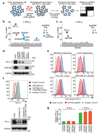
Figure 2. CMTM6 shows functional specificity for PD-L1
a. PD-L1 is readily detected in association with CMTM6. Immunoprecipitation of CMTM6 (left panel) or PD-L1 (right panel) from digitonin lysates of IFN-γ treated MDA-MB-231 cells. Analysis by immunoblot. Lysate = 5% of input. Experiments performed twice. b. Interaction of CMTM6 with PD-L1 is detergent-sensitive. Cells were lysed in 1% digitonin (Dig) and adjusted to the indicated detergent concentrations prior to immunoprecipitation of PD-L1. Experiment performed twice. c. PD-L1 and CMTM6 co-localise at the plasma membrane. PD-L1 knockout, CMTM6 knockout and parental MDA-MB-231 Cas9 cells were fixed, immunostained for PD-L1 and CMTM6 and analysed by confocal microscopy. Scale bar: 10μm. d&e. Quantitative plasma membrane profiling. d. Experimental workflow e. Scatterplots display pairwise comparisons between parental MDA-MB-231 Cas9, CMTM6 sgRNA1 and CMTM6 sgRNA2-expressing cells. Experiment performed in triplicate. q-values determined using LIMMA with Benjamini-Hochberg adjustment for multiple testing.
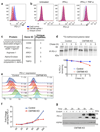
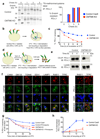
Figure 3. CMTM6 is required for efficient endocytic recycling of PD-L1
a. IFN-γ treated CMTM6 knockout or parental WM-852 Cas9 cells were pulse-labelled with 35S-methionine/cysteine for 30 min, chased at 37°C and PD-L1 immunoprecipitated from detergent lysates at the indicated times. Eluates were split and incubated ± Endoglycosidase-H. Scans quantitated in ImageJ. Representative of 3 experiments. b-e. Cell surface PD-L1 is targeted for degradation in the absence of CMTM6. b&c. IFN-γ treated WM-852 cells were treated as described (b). Representative of six experiments (see Extended Data Fig. 7a & 9a). d&e. Cell surface IP (CS-IP). IFN-γ treated WM-852 cells were treated as described (d) and PD-L1 immunoprecipitates analysed by immunoblot. f. CMTM6 and PD-L1 are identified in recycling endosomes. MDA-MB-231 cells were fixed and immunostained for CMTM6 (large panel) or PD-L1 (right panel) plus markers of the ER (calnexin (CANX)), Golgi (GM130 and TGN46), early endosome (EEA1), late endosome/lysosome (LAMP1) or recycling endosome (RAB11 and transferrin receptor (TFRC)). Scale bar: 10μm. g&h. PD-L1 recycling is impaired in the absence of CMTM6. g. IFN-γ treated WM-852 cells were labelled with an unconjugated PD-L1-specific antibody before incubation at 37°C ± primaquine (described in Extended Data Fig. 8a). Remaining antibody-labelled surface PD-L1 was detected with an AF647-conjugated anti-mouse antibody and analysed by flow cytometry. h. PD-L1 recycling in IFN-γ-treated WM-852 cells (assay described in Extended Data Fig. 8c). Graph shows the proportion of internalised antibody-labelled PD-L1 recycled at each time-point. g&h. Mean and s.e.m. from 3 experiments.
Figure 4. CMTM6 regulates tumour-specific T cell activity by regulating PD-L1 levels
a-c. CMTM6 protects PD-L1 from lysosome-mediated degradation. **a.**35S-methionine/cysteine pulse-chase in IFN-γ treated WM-852 cells (as in Figure 3a) ± 50nM Concanamycin-A (ConA) as indicated b. Quantitation of a in ImageJ. c. Cell-surface IP in CMTM6 knockout cells (see Figure 3d/e). Incubation at 37°C ± 50nM ConA or 50μM Chloroquine d. MDA-MB-231 cells expressing CMTM6-targeting or control shRNAs were incubated ± 50nM ConA for 16 h before analysis by immunoblot e. CMTM6 knockout MDA-MB-231 cells were fixed and immunostained for PD-L1 following 16 h incubation ± ConA. Scale bar: 10μm. f&g. CMTM6 regulates the anti-tumour activity of antigen-specific CTLs f. Intracellular staining for perforin or TNF-α in NY-ESO-192-100 CTLs following 24 h co-culture with CMTM6 sgRNA, PD-L1 sgRNA or control vector transduced LM-MEL-53 Cas9 cells g. IFN-γ and Il-2 levels in supernatants from 3 day co-cultures of NY-ESO-192-100 CTLs and LM-MEL-53 cells f&g. Where indicated, 10μg/ml nivolumab (anti-PD-1) was added to the co-culture. Results are triplicates (mean + s.e.m.) * p < 0.05, ** p < 0.01, unpaired two-tailed t-test. **h&i**. C57BL/6 mice injected subcutaneously with 105 B16-OVA cells transduced with a CMTM6-targeting or control shRNA. **I.** Kaplan-Meier survival curve shows pooled data from 3 experiments (23/24 mice per group). Survival endpoint = tumour >100mm2. Mantel-Cox test. j. Growth of CMTM6-deficient and control tumours, 9 mice per group. Black line indicates mean tumour size. Data from all experiments shown in Extended Data Fig. 10. j. CMTM6 (orange) binds PD-L1 (green) and maintains its cell surface expression. In the absence of CMTM6, endocytosed PD-L1 is rerouted for lysosomal degradation.
Comment in
- Novel regulators of PD-L1 expression in cancer: CMTM6 and CMTM4-a new avenue to enhance the therapeutic benefits of immune checkpoint inhibitors.
Imamovic D, Vranic S. Imamovic D, et al. Ann Transl Med. 2017 Dec;5(23):467. doi: 10.21037/atm.2017.09.32. Ann Transl Med. 2017. PMID: 29285500 Free PMC article. No abstract available. - CMTM6 stabilizes PD-L1 expression and refines its prognostic value in tumors.
Mamessier E, Birnbaum DJ, Finetti P, Birnbaum D, Bertucci F. Mamessier E, et al. Ann Transl Med. 2018 Feb;6(3):54. doi: 10.21037/atm.2017.11.26. Ann Transl Med. 2018. PMID: 29610746 Free PMC article. No abstract available.
Similar articles
- CMTM6, a potential immunotherapy target.
Liang J, Li S, Li W, Rao W, Xu S, Meng H, Zhu F, Zhai D, Cui M, Xu D, Cai J, Zhang B. Liang J, et al. J Cancer Res Clin Oncol. 2022 Jan;148(1):47-56. doi: 10.1007/s00432-021-03835-9. Epub 2021 Nov 16. J Cancer Res Clin Oncol. 2022. PMID: 34783871 Review. - Identification of CMTM6 and CMTM4 as PD-L1 protein regulators.
Mezzadra R, Sun C, Jae LT, Gomez-Eerland R, de Vries E, Wu W, Logtenberg MEW, Slagter M, Rozeman EA, Hofland I, Broeks A, Horlings HM, Wessels LFA, Blank CU, Xiao Y, Heck AJR, Borst J, Brummelkamp TR, Schumacher TNM. Mezzadra R, et al. Nature. 2017 Sep 7;549(7670):106-110. doi: 10.1038/nature23669. Epub 2017 Aug 16. Nature. 2017. PMID: 28813410 Free PMC article. - Hsc70 promotes anti-tumor immunity by targeting PD-L1 for lysosomal degradation.
Xu X, Xie T, Zhou M, Sun Y, Wang F, Tian Y, Chen Z, Xie Y, Wu R, Cen X, Zhou J, Hou T, Zhang L, Huang C, Zhao Q, Wang D, Xia H. Xu X, et al. Nat Commun. 2024 May 18;15(1):4237. doi: 10.1038/s41467-024-48597-3. Nat Commun. 2024. PMID: 38762492 Free PMC article. - Construction of stable membranal CMTM6-PD-L1 full-length complex to evaluate the PD-1/PD-L1-CMTM6 interaction and develop anti-tumor anti-CMTM6 nanobody.
Jia XM, Long YR, Yu XL, Chen RQ, Gong LK, Geng Y. Jia XM, et al. Acta Pharmacol Sin. 2023 May;44(5):1095-1104. doi: 10.1038/s41401-022-01020-3. Epub 2022 Nov 22. Acta Pharmacol Sin. 2023. PMID: 36418428 Free PMC article. - Regulation of PD-L1: a novel role of pro-survival signalling in cancer.
Chen J, Jiang CC, Jin L, Zhang XD. Chen J, et al. Ann Oncol. 2016 Mar;27(3):409-16. doi: 10.1093/annonc/mdv615. Epub 2015 Dec 17. Ann Oncol. 2016. PMID: 26681673 Review.
Cited by
- Post-translational regulations of PD-L1 and PD-1: Mechanisms and opportunities for combined immunotherapy.
Dai X, Gao Y, Wei W. Dai X, et al. Semin Cancer Biol. 2022 Oct;85:246-252. doi: 10.1016/j.semcancer.2021.04.002. Epub 2021 Apr 5. Semin Cancer Biol. 2022. PMID: 33831533 Free PMC article. Review. - CMTM4 is required for IL-17A signaling.
[No authors listed] [No authors listed] Nat Immunol. 2022 Nov;23(11):1525-1526. doi: 10.1038/s41590-022-01344-6. Nat Immunol. 2022. PMID: 36316477 No abstract available. - CMTM4 is a subunit of the IL-17 receptor and mediates autoimmune pathology.
Knizkova D, Pribikova M, Draberova H, Semberova T, Trivic T, Synackova A, Ujevic A, Stefanovic J, Drobek A, Huranova M, Niederlova V, Tsyklauri O, Neuwirth A, Tureckova J, Stepanek O, Draber P. Knizkova D, et al. Nat Immunol. 2022 Nov;23(11):1644-1652. doi: 10.1038/s41590-022-01325-9. Epub 2022 Oct 21. Nat Immunol. 2022. PMID: 36271145 Free PMC article. - Mutual regulation of PD-L1 immunosuppression between tumor-associated macrophages and tumor cells: a critical role for exosomes.
Wang B, Cheng D, Ma D, Chen R, Li D, Zhao W, Fang C, Ji M. Wang B, et al. Cell Commun Signal. 2024 Jan 9;22(1):21. doi: 10.1186/s12964-024-01473-5. Cell Commun Signal. 2024. PMID: 38195554 Free PMC article. Review. - PD-L1 deglycosylation promotes its nuclear translocation and accelerates DNA double-strand-break repair in cancer.
Shu Z, Dwivedi B, Switchenko JM, Yu DS, Deng X. Shu Z, et al. Nat Commun. 2024 Aug 9;15(1):6830. doi: 10.1038/s41467-024-51242-8. Nat Commun. 2024. PMID: 39122729 Free PMC article.
References
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. - PubMed
- Kataoka K, et al. Aberrant PD-L1 expression through 3'-UTR disruption in multiple cancers. Nature. 2016;534:402–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 101835/WT_/Wellcome Trust/United Kingdom
- 20097/CRUK_/Cancer Research UK/United Kingdom
- T32 HG000044/HG/NHGRI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- A20097/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials