Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study - PubMed (original) (raw)
Clinical Trial
. 2017 Sep 14;3(9):e172411.
doi: 10.1001/jamaoncol.2017.2411. Epub 2017 Sep 14.
Peter H O'Donnell 2, Christophe Massard 3, Hendrik-Tobias Arkenau 4, Terence W Friedlander 5, Christopher J Hoimes 6, Jae Lyun Lee 7, Michael Ong 8, Srikala S Sridhar 9, Nicholas J Vogelzang 10, Mayer N Fishman 11, Jingsong Zhang 11, Sandy Srinivas 12, Jigar Parikh 13, Joyce Antal 14, Xiaoping Jin 14, Ashok K Gupta 14, Yong Ben 15, Noah M Hahn 16
Affiliations
- PMID: 28817753
- PMCID: PMC5824288
- DOI: 10.1001/jamaoncol.2017.2411
Clinical Trial
Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results From a Phase 1/2 Open-label Study
Thomas Powles et al. JAMA Oncol. 2017.
Abstract
Importance: The data reported herein were accepted for assessment by the US Food and Drug Administration for Biologics License Application under priority review to establish the clinical benefit of durvalumab as second-line therapy for locally advanced or metastatic urothelial carcinoma (UC), resulting in its recent US approval.
Objective: To report a planned update of the safety and efficacy of durvalumab in patients with locally advanced/metastatic UC.
Design, setting, and participants: This is an ongoing phase 1/2 open-label study of 191 adult patients with histologically or cytologically confirmed locally advanced/metastatic UC whose disease had progressed on, were ineligible for, or refused prior chemotherapy from 60 sites in 9 countries as reported herein.
Intervention: Patients were administered durvalumab intravenous infusion, 10 mg/kg every 2 weeks, for up to 12 months or until progression, starting another anticancer therapy, or unacceptable toxic effects.
Main outcomes and measures: Primary end points were safety and confirmed objective response rate (ORR) per blinded independent central review (Response Evaluation Criteria In Solid Tumors [RECIST], version 1.1).
Results: A total of 191 patients with UC had received treatment. As of October 24, 2016 (90-day update), the median follow-up was 5.78 months (range, 0.4-25.9 months). The median age of patients was 67.0 years and most were male (136 [71.2%]) and white (123 [71.1%]). All patients had stage 4 disease, and 190 (99.5%) had prior anticancer therapy (182 [95.3%] postplatinum). The ORR was 17.8% (34 of 191; 95% CI, 12.7%-24.0%), including 7 complete responses. Responses were early (median time to response, 1.41 months), durable (median duration of response not reached), and observed regardless of programmed cell death ligand-1 (PD-L1) expression (ORR, 27.6% [n = 27; 95% CI, 19.0%-37.5%] and 5.1% [n = 4; 95% CI, 1.4%-12.5%] in patients with high and low or negative expression of PD-L1, respectively). Median progression-free survival and overall survival were 1.5 months (95% CI, 1.4-1.9 months) and 18.2 months (95% CI, 8.1 months to not estimable), respectively; the 1-year overall survival rate was 55% (95% CI, 44%-65%), as estimated by Kaplan-Meier method. Grade 3/4 treatment-related adverse events (AEs) occurred in 13 patients (6.8%); grade 3/4 immune-mediated AEs occurred in 4 patients (2.1%); and treatment-related AEs led to discontinuation of 3 patients (1.6%), 2 of whom had immune-mediated AEs that led to death (autoimmune hepatitis and pneumonitis).
Conclusions and relevance: Durvalumab, 10 mg/kg every 2 weeks, demonstrates favorable clinical activity and an encouraging and manageable safety profile in patients with locally advanced/metastatic UC.
Trial registration: clinicaltrials.gov Identifier: NCT01693562.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Powles reports consulting fees from Genentech/Roche, Bristol-Myers Squibb, Merck, Novartis, and AstraZeneca, and research funding from Genentech/Roche and AstraZeneca/MedImmune. Dr O’Donnell reports advisory board fees from Genentech, Merck, Novartis, Dendreon, Algeta ASA, Inovio, AstraZeneca, Janssen, and Bayer, and research funding to his institution from Boehringer Ingelheim, Genentech, Merck, AstraZeneca/MedImmune, Janssen, and Acerta Pharma; in addition, Dr O’Donnell has a pending patent for a genomic prescribing system. Dr Massard reports advisory board and speaker honoraria from Amgen, Astellas, AstraZeneca, Bayer, Celgene, Genentech, Ipsen, Janssen, Lilly, Novartis, Pfizer, Roche, Sanofi, and Orion. Dr Friedlander reports advisory board fees from Roche Genentech and Pfizer, speaker honoraria from Sanofi Aventis, Astellas Medivation, Dendreon, EMD Serono, and Prometheus, and research support from Janssen, Novartis, and ImClone. Dr Hoimes reports research grants from Merck and advisory board fees from Bristol-Myers Squibb, Roche-Genentech, and Prometheus. Dr Lee reports speaker honoraria from Pfizer and Astellas and advisory role fees from Astellas, Eisai Korea, and AstraZeneca. Dr Ong reports consulting fees from AstraZeneca/MedImmune, Bristol-Myers Squibb, Merck, and Genentech/Roche. Dr Sridhar reports consulting fees from AstraZeneca/MedImmune, Bristol-Myers Squibb, Genentech/Roche, and Pfizer. Dr Vogelzang reports consulting fees and honorarium from AstraZeneca. Dr Fishman reports research support to his institution from AstraZeneca/MedImmune, Bristol-Myers Squibb, Merck, Genentech/Roche, Pfizer, Prometheus, Acceleron, Alkermes, Exelixis, and Eisai, speaker bureau honoraria from Pfizer and Exelixis, and consulting fees from Alkermes and Eisai. Dr. Zhang reports research support to his institution from Astellas/Medivation, AstraZeneca, and Bayer, speaker bureau honoraria from Sanofi Genzyme and AstraZeneca, and consulting fees from Sanofi Genzyme. Dr Srinivas reports consulting fees from AstraZeneca/MedImmune, Bristol-Myers Squibb, Genentech/Roche, and Pfizer. Ms Antal and Drs Jin and Gupta are full-time employees of MedImmune, and Dr Ben is a full-time employee of AstraZeneca. Ms Antal and Drs Jin, Gupta and Ben report stock ownership from AstraZeneca. Dr Gupta also reports stock ownership from Bristol-Myers Squibb. Dr Hahn reports research support to his institution and consulting fees from AstraZeneca/MedImmune, Bristol-Myers Squibb, Merck, Genentech/Roche, and OncoGenex, research support to his institution from Acerta, Mirati Pharmaceuticals, and Novartis and consulting fees from Inovio Pharmaceuticals. No other disclosures are reported.
Figures
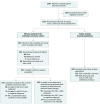
Figure 1.. Study Profile
The overall population included 17 different tumor-specific cohorts; 2367 patients were screened, of whom 1355 were screen failures. ≥2L indicates second-line or greater; Q2W, every 2 weeks; PD-L1, programmed cell death ligand-1. aA large portion of screen failures were based on their PD-L1 status and eligibility criteria requiring a specific PD-L1 status for enrollment. bThese included 6 patients from the dose-escalation phase of the study. cPD-L1 expression status was unknown (owing to insufficient tumor in biopsy) or unavailable (as testing had not been processed at data cutoff) for 14 patients.
Figure 2.. Antitumor Activity and Kaplan-Meier Estimates of Overall Survival in the Cohort With Urothelial Carcinoma by PD-L1 Expression Status
Programmed cell death ligand-1 (PD-L1) expression status was unknown (owing to insufficient tumor in biopsy) or unavailable (as testing had not been processed at data cutoff) for 14 patients. A, Time to response and duration of response (DoR) by blinded independent central review (BICR). B, Best percentage change from baseline in tumor size by BICR (patients with target lesions at baseline and ≥1 postbaseline scan). One patient in the PD-L1 high subgroup with confirmed complete response was excluded from the figure owing to lack of a target lesion at baseline per BICR. For patients with lymph nodes included in their target lesions, complete response may not equate with a 100% decrease from baseline according to Response Evaluation Criteria in Solid Tumors, version 1.1. C, Overall survival (OS). Given limited follow-up, OS data were considered immature at the time of data cutoff. CR indicates complete response; NE, not evaluable; neg, negative; PR, partial response.
Comment in
- Re: Efficacy and Safety of Durvalumab in Locally Advanced or Metastatic Urothelial Carcinoma: Updated Results from a Phase 1/2 Open-Label Study.
Chang SS. Chang SS. J Urol. 2018 May;199(5):1110-1112. doi: 10.1016/j.juro.2018.02.033. Epub 2018 Feb 17. J Urol. 2018. PMID: 29677898 No abstract available.
Similar articles
- Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial.
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA, van der Heijden MS, Dreicer R, Srinivas S, Retz MM, Joseph RW, Drakaki A, Vaishampayan UN, Sridhar SS, Quinn DI, Durán I, Shaffer DR, Eigl BJ, Grivas PD, Yu EY, Li S, Kadel EE 3rd, Boyd Z, Bourgon R, Hegde PS, Mariathasan S, Thåström A, Abidoye OO, Fine GD, Bajorin DF; IMvigor210 Study Group. Balar AV, et al. Lancet. 2017 Jan 7;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2. Epub 2016 Dec 8. Lancet. 2017. PMID: 27939400 Free PMC article. Clinical Trial. - Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial.
Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, Britten CD, Dirix L, Lee KW, Taylor M, Schöffski P, Wang D, Ravaud A, Gelb AB, Xiong J, Rosen G, Gulley JL, Apolo AB. Patel MR, et al. Lancet Oncol. 2018 Jan;19(1):51-64. doi: 10.1016/S1470-2045(17)30900-2. Epub 2017 Dec 5. Lancet Oncol. 2018. PMID: 29217288 Free PMC article. Clinical Trial. - Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study.
Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J, Chouaid C, Bidoli P, Wheatley-Price P, Park K, Soo RA, Huang Y, Wadsworth C, Dennis PA, Rizvi NA; ATLANTIC Investigators. Garassino MC, et al. Lancet Oncol. 2018 Apr;19(4):521-536. doi: 10.1016/S1470-2045(18)30144-X. Epub 2018 Mar 12. Lancet Oncol. 2018. PMID: 29545095 Free PMC article. Clinical Trial. - Programmed Death 1 and Programmed Death Ligand 1 Inhibitors in Advanced and Recurrent Urothelial Carcinoma: Meta-analysis of Single-Agent Studies.
Tafuri A, Smith DD, Cacciamani GE, Cole S, Shakir A, Sadeghi S, Vogelzang NJ, Quinn D, Gill PS, Gill IS. Tafuri A, et al. Clin Genitourin Cancer. 2020 Oct;18(5):351-360.e3. doi: 10.1016/j.clgc.2020.01.004. Epub 2020 Jan 31. Clin Genitourin Cancer. 2020. PMID: 32146152 Free PMC article. Review. - The Clinical Safety and Efficacy of Targeted PD-L1 Therapy with Durvalumab in Solid Tumors.
Chen M, Jiang J, Chen J, Wang M, Lu Y, Liu L, Zhao L, Wang L. Chen M, et al. Curr Drug Targets. 2023;24(7):584-598. doi: 10.2174/1389450124666230330101651. Curr Drug Targets. 2023. PMID: 36998143 Review.
Cited by
- Small-molecule inhibitors, immune checkpoint inhibitors, and more: FDA-approved novel therapeutic drugs for solid tumors from 1991 to 2021.
Wu Q, Qian W, Sun X, Jiang S. Wu Q, et al. J Hematol Oncol. 2022 Oct 8;15(1):143. doi: 10.1186/s13045-022-01362-9. J Hematol Oncol. 2022. PMID: 36209184 Free PMC article. Review. - TERT promoter mutations and other prognostic factors in patients with advanced urothelial carcinoma treated with an immune checkpoint inhibitor.
de Kouchkovsky I, Zhang L, Philip EJ, Wright F, Kim DM, Natesan D, Kwon D, Ho H, Ho S, Chan E, Porten SP, Wong AC, Desai A, Huang FW, Chou J, Oh DY, Pruthi RS, Fong L, Small EJ, Friedlander TW, Koshkin VS. de Kouchkovsky I, et al. J Immunother Cancer. 2021 May;9(5):e002127. doi: 10.1136/jitc-2020-002127. J Immunother Cancer. 2021. PMID: 33980590 Free PMC article. - Latin American Consensus for the Evaluation and Treatment of Patients With Metastatic/Locally Advanced Urothelial Carcinoma.
Manneh Kopp R, Galanternik F, Schutz FA, Kater F, Ramos-Esquivel A, Neciosup S, Sobrevilla-Moreno N, Bernal Vaca L, Ibatá-Bernal L, Martínez-Rojas S, Bourlon MT. Manneh Kopp R, et al. JCO Glob Oncol. 2024 Jan;10:e2300244. doi: 10.1200/GO.23.00244. JCO Glob Oncol. 2024. PMID: 38271646 Free PMC article. - The rapidly changing treatment landscape of first-line advanced urothelial cancer (aUC) or metastatic urothelial cancer (mUC).
Aslanova M, Yu EM, Aragon-Ching JB. Aslanova M, et al. Explor Target Antitumor Ther. 2024;5(4):971-980. doi: 10.37349/etat.2024.00258. Epub 2024 Jul 29. Explor Target Antitumor Ther. 2024. PMID: 39280249 Free PMC article. - The efficacy and safety of chemotherapy with or without anti-PD-1 for the first-line treatment of advanced urothelial carcinoma.
Han F, Wu Z, Chen J, Liu M, Hu Y. Han F, et al. Cancer Med. 2023 Dec;12(23):21129-21137. doi: 10.1002/cam4.6671. Epub 2023 Nov 21. Cancer Med. 2023. PMID: 37990780 Free PMC article.
References
- Bellmunt J, Théodore C, Demkov T, et al. . Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27(27):4454-4461. - PubMed
- Bellmunt J, Mullane SA, Werner L, et al. . Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26(4):812-817. - PubMed
- Patel SP, Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14(4):847-856. - PubMed
- Ilie M, Hofman V, Dietel M, Soria JC, Hofman P. Assessment of the PD-L1 status by immunohistochemistry: challenges and perspectives for therapeutic strategies in lung cancer patients. Virchows Arch. 2016;468(5):511-525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials