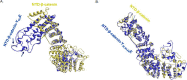Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through β-catenin - PubMed (original) (raw)
Janus kinase 3 regulates adherens junctions and epithelial mesenchymal transition through β-catenin
Jayshree Mishra et al. J Biol Chem. 2017.
Abstract
Compromise in adherens junctions (AJs) is associated with several chronic inflammatory diseases. We reported previously that Janus kinase 3, a non-receptor tyrosine kinase, plays a crucial role in AJ formation through its interaction with β-catenin. In this report, we characterize the structural determinants responsible for Jak3 interactions with β-catenin and determine the functional implications of previously unknown tyrosine residues on β-catenin phosphorylated by Jak3. We demonstrate that Jak3 autophosphorylation was the rate-limiting step during Jak3 trans-phosphorylation of β-catenin, where Jak3 directly phosphorylated three tyrosine residues, viz. Tyr30, Tyr64, and Tyr86 in the N-terminal domain (NTD) of β-catenin. However, prior phosphorylation of β-catenin at Tyr654 was essential for further phosphorylation of β-catenin by Jak3. Interaction studies indicated that phosphorylated Jak3 bound to phosphorylated β-catenin with a dissociation constant of 0.28 μm, and although both the kinase and FERM (Band 41, ezrin, radixin, and moesin) domains of Jak3 interacted with β-catenin, the NTD domain of β-catenin facilitated its interactions with Jak3. Physiologically, Jak3-mediated phosphorylation of β-catenin suppressed EGF-mediated epithelial-mesenchymal transition and facilitated epithelial barrier functions by AJ localization of phosphorylated β-catenin through its interactions with α-catenin. Moreover, loss of Jak3-mediated phosphorylation sites in β-catenin abrogated its AJ localization and compromised epithelial barrier functions. Thus, we not only characterize Jak3 interaction with β-catenin but also demonstrate the mechanism of molecular interplay between AJ dynamics and EMT by Jak3-mediated NTD phosphorylation of β-catenin.
Keywords: JAK; adherens junction; barrier functions; epithelial–mesenchymal transition (EMT); mucosal immunology; protein structure function; β-catenin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Figures
Figure 1.
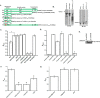
Recombinant Jak3 trans-phosphorylates the adherent junction protein β-catenin. A and B, the non-phosphorylated and phosphorylated forms of the β-catenin protein were produced using BL21 and TKX1 expression system as reported before (20) and were confirmed through IB using phosphotyrosine (A, top panel), β-catenin (A, bottom panel) and Jak3 (B) antibodies. C, changes in tyrosine trans-phosphorylation of β-catenin by Jak3 were detected using a β-catenin–coated 96-well microtiter plate and induction of phosphorylation by addition of activated (P) Jak3 (US Patent 9739779) (14, 20, 49), where P-Jak3-WT alone and β-catenin alone were taken as controls. The phosphorylation was detected as reported before (19, 20). Curve fitting was done as reported before (19) using the Hyperbol-fit program in MicroCal Origin to calculate _t_½. D, similar experiments were performed as in C but in the presence of the Jak3 inhibitor CP-690505 and with a fixed reaction time of 5 min. E, comparison of _t_½ of Jak3-mediated transphosphorylation of villin (20), p52ShcA (19), and β-catenin. F, HT-29 CL-19a cells were treated with IL-2 in the presence or absence of the Jak3 inhibitor CP-690550 for the indicated time, followed by IB using cell lysates from treated cells and the indicated antibodies. G, the binding kinetics of Jak3 interactions with β-catenin were determined by a pairwise binding assay, followed by analysis of the data using Hyperbol-fit as reported before (19). OD, optical density. A, B, and F, blots are representative of n = 3 experiments. C, D, and G, values are mean ± S.E. *, p < 0.05, indicating statistically significant differences from the control; n = 3 experiments.
Figure 2.
Characterization of structural determinants responsible for Jak3 interactions with β-catenin. A, schematic of His–β-catenin–WT and mutants. B, His-tagged protein β-catenin–WT and these mutants were expressed and purified as in Fig. 1_A_ and confirmed through Western blot analysis of the expressed proteins using anti-His antibody. Arrows indicate recombinant protein expression. C, transphosphorylation experiments were performed using the WT and indicated mutants of β-catenin as substrate. Phosphorylation was induced as in Fig. 1_C_, where P-Jak3-WT alone or β-catenin or its mutants alone were used as controls. D, direct interactions between Jak3-WT and β-catenin or its mutants were determined as in C, where direct interactions between β-catenin and Jak3 mutants were determined as in Fig. 1_G_ using BSA and villin as negative and positive controls, respectively. E, Western blot analysis of the indicated purified recombinant proteins of β-catenin were done using β-catenin antibody. F, direct interactions between β-catenin–WT and the indicated mutants of Jak3 were determined as in D. G, direct interactions between Jak3-WT and the indicated mutants of β-catenin were determined as in D. The blots in B and E are representative of n = 3 experiments. C, D, F, and G, values are mean ± S.E. *, p < 0.05, indicating statistically significant differences from β-catenin–WT (C, D, and G) or Jak3-WT (F); n = 3 experiments.
Figure 3.
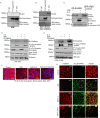
Jak3 phosphorylation of Tyr30, Tyr64, and Tyr86 in β-catenin facilitates barrier functions. A, Western blot analyses of immunoprecipitates from the cell lysates of stably transfected HT-29 Cl-19A cells were done for the expression of HA-tagged β-catenin or its indicated mutants. B, stably transfected cells of HA-tagged β-catenin and its mutant from A were treated with IL-2 (50 units/ml) only or with CP-690550 (100 mg/ml). Cell lysates were subjected to IP, followed by IB using the indicated antibodies as reported before (22). C, similar experiments were performed as in B but using stably transfected cells of HA-NTD or its mutant. D, similar experiments were performed as in B but using stably transfected cells of HA–β-catenin–Y30F,Y64F,Y86F. E, normal epithelial cells, FHC CRL-1813, were transiently transfected with cDNA constructs containing HA-tagged β-catenin–WT or the indicated mutant. IP followed by IB analysis were done using the indicated antibodies and cell lysates from the transfected cells to confirm comparable expression of transfected proteins. F and G, control (untransfected), HA–β-catenin-, and HA–β-catenin–Y41F,Y61F,Y81F–expressing FHC CRL-1813 (F) and HT-29 Cl-19a (G) cells were cultured in a 6-transwell plate to confluence, and TEER was measured. Mean TEER values (n = 6) are shown. *, p < 0.05, indicating statistically significant differences from β-catenin–WT–transfected cells. H, AJ localization of β-catenin was determined using IFM in confluent HT-29 Cl-19a cells stably transfected with either HA-β-catenin or HA–β-catenin–Y30F,Y64F,Y86F using anti-HA antibody. Green indicates localization of HA-tagged proteins. Note that mutation in β-catenin at Y30F,Y64F,Y86F disrupts its AJ localization, as denoted by punctate green staining. Scale bar = 14 μm. I, IHC staining for the localization of Tyr654-phosphorylated β-catenin in colonic mucosa of WT and Jak3 KO mice was performed using the protocol described under “Experimental procedures.” Red arrows indicate the differences in localization of Tyr654-phosphorylated β-catenin in the absence of Jak3. Note that lack of Jak3 results in loss of localization of Tyr654-phosphorylated β-catenin at cellular junctions. A–E, the blots shown are representative (n = 3). H and I, the data shown are representative (n = 6/group).
Figure 4.
Phosphorylation of Tyr30, Tyr64, and Tyr86 in β-catenin by Jak3 suppresses EMT. A and B, HT-29 CL19a cells were stimulated with either EGF (100 μg/ml) alone (A) to facilitate Tyr654 phosphorylation of β-catenin or in the presence of the Src inhibitor J67242 (50 m
m
) (B) to prevent Tyr654 phosphorylation of β-catenin. Association of Jak3 with Tyr654-phosphorylated β-catenin was determined by co-IP using the indicated antibodies. IB using β-catenin antibody was done for input control. C, HT-29 CL19a cells expressing either HA–β-catenin or HA–β-catenin–Y30F,Y64F,Y86F were stimulated either with EGF (100 μg/ml) alone or in the presence of IL-2 (50 units/ml). β-Catenin association either with α-catenin as an indicator of AJ integrity or with Hif1α as an indicator of mesenchymal transition was determined using co-IP, followed by IB with indicated antibodies. D and E, EMT was induced in normal epithelial cells, CRL-1813 (D), and in transformed epithelial cells, HT-29 Cl-19a (E), by stimulation with EGF-mediated activation of Src kinase as reported before (23), and Jak3-mediated suppression of EMT was determined through simultaneous activation of the cells by EGF and IL-2. Induction of EMT was confirmed by IB for the EMT marker N-cadherin and co-IP for β-catenin association with Hifα. AJ stabilization was confirmed through co-IP for β-catenin association with α-catenin. IB using β-catenin indicates input control, and using β-actin indicates loading control. A–E, blots are representative (n = 3 experiments). F and G, HT-29 CL19a cells were stimulated either with EGF alone in the presence of IL-2, or in the presence of IL-2 + the Jak3 inhibitor tofacitinib, and AJ integrity was determined through confocal microscopy analysis of either α-catenin localization alone (F) or through its co-localization with Tyr654-phosphorylated β-catenin (G). Representative images are shown (n = 6 experiments). Green indicates Tyr654-phosphorylated β-catenin, and red indicates α-catenin. Scale bars = 14 μm.
Figure 5.
Loss of Tyr30, Tyr64, and Tyr86 phosphorylation in the NTD domain of β-catenin facilitates EMT. A and B, HT-29 Cl-19a cells were either left unstimulated (control) or stimulated with EGF and/or IL-2 alone or in the presence of Src-kinase inhibitor (SRCi) or Jak3 kinase inhibitor (Tof). Flow cytometry estimation of the cells was performed as detailed under “Experimental procedures” to determine expression of the EMT marker N-cadherin. Note that the top panels in A confirm EGF stimulation of the EMT marker N-cadherin, whereas the bottom panels show reversal of these effects by IL-2–mediated activation of Jak3. B, there was a 10-fold increase in expression of the EMT marker by EGF that was suppressed almost 3-fold by IL-2 activation of Jak3. A, scatterplots are representative (n = 3 experiments). B, plots are the average of three experiments. **, indicating a statistically significant difference with control (for EGF) and with IL-2+EGF (for IL-2+EGF+Tof). C, stably transfected cells of Jak3-mediated phospho-mutants (Y30F,Y64F,Y86F) of β-catenin in HT-29 Cl-19a cells were either taken unstimulated (control) or stimulated with EGF alone or in the presence of IL-2. Cell lysates were prepared and subjected to either IB alone or IP followed by IB using the indicated antibodies. Although association of β-catenin with α-catenin determined AJ stabilization, expression of the EMT marker N-cadherin and β-catenin association with Hifα determined the induction of EMT. IB using β-catenin antibody demonstrated input controls. Blots are representative (n = 3 experiments). D, deidentified and discarded colonic tissue samples of human subjects either healthy or having colonic polyps were immunostained either with the EMT marker N-cadherin or our newly identified EMT suppressor Jak3 using procedures detailed under “Experimental procedures.” Images were acquired using a Nikon C1-plus laser confocal microscope, and representative images are shown from each group (n = 5). Note the significantly decreased expression and altered localization of the EMT suppressor Jak3 in colonic polyps (right panels) compared with healthy tissues (left panels). Also note the increased expression of the EMT marker N-cadherin exclusively in colonic polyps, where Jak3 expression was markedly decreased. Scale bars = 550 μm.
Figure 6.
Molecular dynamics of Jak3-mediated phosphorylation sites in β-catenin. A and B, full-length β-catenin was modeled using the Phyre2 modeling server, and structural shifts because of phosphorylation were reconstructed by a phospho-mimetic approach through 3D mutant substitutions of Tyr to Glu either at Y654E (A) or at Y30E,Y64E,Y86E (B). Structural prediction was done using primary simulations through Phyre2. Energy minimization was done by dynamic simulations using CHARMM Forcefield (Chemistry at Harvard Macromolecular Mechanics) to stabilize the phospho-mutant structures. The conformational shifts in the NTD of β-catenin induced by the Tyr-to-Glu substitutions were evaluated by superimposition of the protein structures and positional determination of residues Ala80, Gly85, and Val166 as markers to evaluate the relative orientation of the NTD of β-catenin. Arrows (gold, β-catenin–WT; blue, β-catenin Y654E) indicate the corresponding positions of Ala80, Gly85, and Val166 in the modeled proteins. Note that superimposed β-catenin–WT (gold) on Y30E,Y64E,Y86E (blue) in B show overlap of the positional markers Gly85 and Val166 in the structure of the protein, indicating a close resemblance in conformation of the NTD of β-catenin–WT and β-catenin–Y30E,Y64E,Y86E and a reversal of orientation from β-catenin–Y654E in A.
Similar articles
- Intestinal breast cancer resistance protein (BCRP) requires Janus kinase 3 activity for drug efflux and barrier functions in obesity.
Mishra J, Simonsen R, Kumar N. Mishra J, et al. J Biol Chem. 2019 Nov 29;294(48):18337-18348. doi: 10.1074/jbc.RA119.007758. Epub 2019 Oct 25. J Biol Chem. 2019. PMID: 31653704 Free PMC article. - Role of Janus kinase 3 in mucosal differentiation and predisposition to colitis.
Mishra J, Verma RK, Alpini G, Meng F, Kumar N. Mishra J, et al. J Biol Chem. 2013 Nov 1;288(44):31795-806. doi: 10.1074/jbc.M113.504126. Epub 2013 Sep 17. J Biol Chem. 2013. PMID: 24045942 Free PMC article. - Adapter protein Shc regulates Janus kinase 3 phosphorylation.
Mishra J, Kumar N. Mishra J, et al. J Biol Chem. 2014 Jun 6;289(23):15951-6. doi: 10.1074/jbc.C113.527523. Epub 2014 May 2. J Biol Chem. 2014. PMID: 24795043 Free PMC article. - Actin filament association at adherens junctions.
Yonemura S. Yonemura S. J Med Invest. 2017;64(1.2):14-19. doi: 10.2152/jmi.64.14. J Med Invest. 2017. PMID: 28373611 Review. - Mucosal Epithelial Jak Kinases in Health and Diseases.
Kumar N, Kuang L, Villa R, Kumar P, Mishra J. Kumar N, et al. Mediators Inflamm. 2021 Mar 16;2021:6618924. doi: 10.1155/2021/6618924. eCollection 2021. Mediators Inflamm. 2021. PMID: 33814980 Free PMC article. Review.
Cited by
- Phosphorylation-Dependent Regulation of WNT/Beta-Catenin Signaling.
Shah K, Kazi JU. Shah K, et al. Front Oncol. 2022 Mar 14;12:858782. doi: 10.3389/fonc.2022.858782. eCollection 2022. Front Oncol. 2022. PMID: 35359365 Free PMC article. Review. - Intestinal breast cancer resistance protein (BCRP) requires Janus kinase 3 activity for drug efflux and barrier functions in obesity.
Mishra J, Simonsen R, Kumar N. Mishra J, et al. J Biol Chem. 2019 Nov 29;294(48):18337-18348. doi: 10.1074/jbc.RA119.007758. Epub 2019 Oct 25. J Biol Chem. 2019. PMID: 31653704 Free PMC article. - Differential expression levels of β-catenin are associated with invasive behavior of both functional and non-functional pituitary neuroendocrine tumor (PitNET).
Taghavi SF, Ghorbani M, Panahi M, Nazem S, Karimi M, Salimi V, Tavakoli-Yaraki M. Taghavi SF, et al. Mol Biol Rep. 2023 Aug;50(8):6425-6434. doi: 10.1007/s11033-023-08523-0. Epub 2023 Jun 16. Mol Biol Rep. 2023. PMID: 37326745 - Mechanistic Role of Jak3 in Obesity-Associated Cognitive Impairments.
Kumar P, Mishra J, Kumar N. Kumar P, et al. Nutrients. 2022 Sep 9;14(18):3715. doi: 10.3390/nu14183715. Nutrients. 2022. PMID: 36145091 Free PMC article. - Low-Grade Ovarian Stromal Tumors with Genetic Alterations of the Wnt/β-Catenin Pathway That Is Crucial in Ovarian Follicle Development and Regulation.
Zhang G, Michener CM, Yang B. Zhang G, et al. Cancers (Basel). 2022 Nov 16;14(22):5622. doi: 10.3390/cancers14225622. Cancers (Basel). 2022. PMID: 36428715 Free PMC article. Review.
References
- Padmanabhan A., Rao M. V., Wu Y., and Zaidel-Bar R. (2015) Jack of all trades: functional modularity in the adherens junction. Curr. Opin. Cell Biol. 36, 32–40 - PubMed
- Mehta S., Nijhuis A., Kumagai T., Lindsay J., and Silver A. (2015) Defects in the adherens junction complex (E-cadherin/β-catenin) in inflammatory bowel disease. Cell Tissue Res. 360, 749–760 - PubMed
- Alves C. H., Pellissier L. P., and Wijnholds J. (2014) The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog. Retin. Eye Res. 40, 35–52 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials