Zika-Virus-Encoded NS2A Disrupts Mammalian Cortical Neurogenesis by Degrading Adherens Junction Proteins - PubMed (original) (raw)
. 2017 Sep 7;21(3):349-358.e6.
doi: 10.1016/j.stem.2017.07.014. Epub 2017 Aug 17.
Guang Song 2, Xuyu Qian 3, Jianbo Pan 4, Dan Xu 5, Hee-Sool Rho 2, Nam-Shik Kim 1, Christa Habela 6, Lily Zheng 7, Fadi Jacob 8, Feiran Zhang 9, Emily M Lee 10, Wei-Kai Huang 11, Francisca Rojas Ringeling 12, Caroline Vissers 13, Cui Li 14, Ling Yuan 14, Koeun Kang 15, Sunghan Kim 15, Junghoon Yeo 15, Yichen Cheng 10, Sheng Liu 4, Zhexing Wen 16, Cheng-Feng Qin 17, Qingfeng Wu 14, Kimberly M Christian 18, Hengli Tang 10, Peng Jin 9, Zhiheng Xu 14, Jiang Qian 4, Heng Zhu 2, Hongjun Song 19, Guo-Li Ming 20
Affiliations
- PMID: 28826723
- PMCID: PMC5600197
- DOI: 10.1016/j.stem.2017.07.014
Zika-Virus-Encoded NS2A Disrupts Mammalian Cortical Neurogenesis by Degrading Adherens Junction Proteins
Ki-Jun Yoon et al. Cell Stem Cell. 2017.
Abstract
Zika virus (ZIKV) directly infects neural progenitors and impairs their proliferation. How ZIKV interacts with the host molecular machinery to impact neurogenesis in vivo is not well understood. Here, by systematically introducing individual proteins encoded by ZIKV into the embryonic mouse cortex, we show that expression of ZIKV-NS2A, but not Dengue virus (DENV)-NS2A, leads to reduced proliferation and premature differentiation of radial glial cells and aberrant positioning of newborn neurons. Mechanistically, in vitro mapping of protein-interactomes and biochemical analysis suggest interactions between ZIKA-NS2A and multiple adherens junction complex (AJ) components. Functionally, ZIKV-NS2A, but not DENV-NS2A, destabilizes the AJ complex, resulting in impaired AJ formation and aberrant radial glial fiber scaffolding in the embryonic mouse cortex. Similarly, ZIKA-NS2A, but not DENV-NS2A, reduces radial glial cell proliferation and causes AJ deficits in human forebrain organoids. Together, our results reveal pathogenic mechanisms underlying ZIKV infection in the developing mammalian brain.
Keywords: Zika virus; adherens junction; cortical neurogenesis; flavivirus; human organoid; human protein microarray; microcephaly; neural stem cell; neuronal migration; radial glial cell.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS: The authors declare no competing financial interests.
Figures
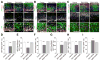
Figure 1. ZIKV-NS2A, but not DENV-NS2A, dysregulates radial glial cells in the embryonic mouse cortex
Embryonic mouse cortex was electroporated at E14.5 to express GFP, GFP and ZIKV-NS2A, or GFP and DENV-NS2A, followed by EdU labeling 2 hr before analysis at E17.5. Sample confocal images of immunostaining for Pax6 (A), Tbr2 (B), Tuj1 (C) and GFP, and staining for EdU and DAPI are shown. Scale bars: 100 μm (A, B, C). The regions in white boxes (top panels) are shown at a higher magnification (bottom panels). Quantifications are also shown (D–I). Values represent mean + SEM (n = 5–7 sections from 3–4 animals; ***: P < 0.001; **: P < 0.01; One-way ANOVA). Also see Figure S1 and S2.
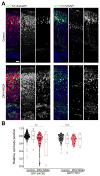
Figure 2. ZIKV-NS2A, but not DENV-NS2A, expression leads to aberrant localization of neurons in the developing mouse cortex
(A) Embryonic mouse cortex was electroporated at E14.5 to express GFP, or GFP and ZIKV-NS2A, followed by analysis at E19.5. Sample confocal images of immunostaining for SATB2, CTIP2 and GFP, and staining for DAPI are shown. Scale bar: 50 μm. (B) Scatter plots and summary of cell body position of GFP+SATB2+ neurons and GFP−CTIP2+ neurons in the mouse cortex at E19.5. The distance of each cell to the apical surface was normalized to the total thickness of the neocortex. Each dot represents one neuron. The box plots show the medians (line), means (square), interquartile ranges (box; 25–75%), and extremes of the distribution (whisker; 99%: upper crosshatch; 1%: lower crosshatch) (n = 8 sections from 4 animals for each condition; ** P < 0.01; n.s. _P_ > 0.1; Student’s t-test).
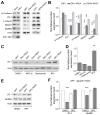
Figure 3. Protein-protein interactomes of ZIKV-NS2A and DENV-NS2A across the human proteome
(A) 143 and 47 direct ZIKV-NS2A and DENV-NS2A interacting proteins were identified in vitro using a protein array containing 20,240 full-length human proteins. Among 143 ZIKV-NS2A interacting proteins, 83 proteins can be visualized in a connected network based on existing literatures (P < 4.91 × 10−13; STRING analysis), whereas the remaining 60 proteins are singletons. ZIKV-NS2A-specific interacting proteins are coded in gray and adhesion-related proteins are highlighted with circles. (B) Sample western blot images of co-IP analysis of HEK293 cells expressing GFP, ZIKV-NS2A and GFP, or DENV and GFP, and analyzed for adherens junction complex components. Histone H3 served as a negative control for Co-IP. Also see Figure S3.
Figure 4. ZIKV-NS2A, but not DENV-NS2A, expression leads to adherens junction complex component degradation
(A–B) Sample western blot images of expression levels of adherens junction complex components in mouse neural progenitors infected with ZIKV, expressing ZIKV-NS2A, or expressing DENV-NS2A (A), and quantifications (B). Data were normalized to that of mock infection for the ZIKV infection condition, or to that of GFP expression alone for the ZIKV-NS2A or DENV-NS2A conditions. Values represent mean + SEM (n = 3 cultures; ***: P < 0.001; *: P < 0.05; Student’s t-test). (C–D) Sample western blot images of expression levels of ZO-1 upon 24 hr treatment of DMSO, MG-132 (20 μM), Delanzomib (60 nM) and BFA (100 nM) in HEK293 cells expressing GFP and ZIKV-NS2A, or GFP alone (C), and quantifications (D). Data were first normalized to actin expression levels and then to the data from expression of GFP alone. Values represent mean + SEM (n = 3 cultures; **: P < 0.01; Student’s t-test). (E–F) Sample western blot images of expression levels of ZO-1 and NUMBL upon 24 hr treatment of DMSO, or BFA (100 nM) of mouse cortical neural progenitors expressing GFP and ZIKV-NS2A, or GFP alone (E), and quantifications (F). Data were normalized to that of actin. Values represent mean + SEM (n = 3 cultures; ***: P < 0.001; Student’s t-test). Also see Figure S4.
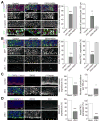
Figure 5. ZIKV-NS2A expression and direct ZIKV infection disrupt the formation of adherens junction complex in the embryonic mouse cortex
(A–B) Embryonic mouse brains were electroporated at E14.5 to express GFP, GFP and ZIKV-NS2A, or GFP and DENV-NS2A, and analyzed at E17.5. Sample confocal images of immunostaining for GFP, β-Catenin (A), or PKCλ and ZO-1 (B), and staining for DAPI under different conditions are shown (left panels). Scale bars: 50 μm. Arrows point to regions with discontinuous AJ formation. Regions in white boxes in (A) are shown at a higher magnification (bottom panels). Quantifications of continuous AJ formation and number of protrusions are also shown (right panels). Values represent mean + SEM (n = 5 sections from 3 animals; ***: P < 0.001; **: P < 0.01; Student’s t-test). (C–D) ZIKV-SZ strain was injected into lateral ventricles of E13.5/E14.5 mice. Similar to (A–B), sample confocal images of immunostaining for ZIKV, β-Catenin and ZO-1, and staining for DAPI at E18.5 (left panels) and quantifications of continuous AJ formation at E18.5 and ventricular protrusions at P3 (right panels) are shown. Values represent mean + SEM (n = 6 sections from 4 animals; ***: P < 0.001; *: P < 0.05; Student’s t-test). Also see Figure S5.
Figure 6. Expression of ZIKV-NS2A, but not DENV-NS2A, reduces proliferation and disrupts adherens junction formation of ventricular radial glial cells in human forebrain organoids
(A) Day 45 forebrain organoids were electroporated to express GFP, GFP and ZIKV-NS2A, or GFP and DENV-NS2A, and analyzed 3 days later (45+3) after pulsing with EdU (10 μM) for 1 hr. Sample confocal images for immunostaining for GFP and PAX6, and staining for EdU and DAPI are shown (top panels). Scale bar: 100 μm. Quantifications of percentages of EdU+GFP+ cells among all GFP+ cells, or GFP+PAX6+EdU+ cells among GFP+PAX6+ cells are also shown (bottom panels). Values represent mean + SEM (n = 10 organoids; ***: P < 0.001; Student’s t-test). (B) Sample confocal images of immunostaining for GFP, PKCλ, and staining for DAPI are shown (left panels). Scale bar: 100 μm. Arrows point to regions with discontinuous AJ formation. Quantifications of AJ continuity based on PKCλ expression are also shown (right panel). Values represent mean + SEM (n = 9 organoids; **: P < 0.01; Student’s t-test). (C) Expression of ZIKV-NS2A, but not DENV-NS2A, or GFP alone, led to a loss of typical radial glia morphology of PAX6+GFP+ cells at 7 days after electroporation (45+7). Sample confocal images of immunostaining for GFP and PAX6, and staining for DAPI (left panels; scale bar: 100 μm) and quantification of percentages of GFP+PAX6+ cells with multipolar morphologies are shown. Values represent mean + SEM (n = 7 organoids; **: P < 0.01; Student’s t-test). Also see Figure S6.
Similar articles
- Zika Virus NS2A-Mediated Virion Assembly.
Zhang X, Xie X, Xia H, Zou J, Huang L, Popov VL, Chen X, Shi PY. Zhang X, et al. mBio. 2019 Oct 29;10(5):e02375-19. doi: 10.1128/mBio.02375-19. mBio. 2019. PMID: 31662457 Free PMC article. - Genetic and biochemical characterizations of Zika virus NS2A protein.
Zhang X, Xie X, Zou J, Xia H, Shan C, Chen X, Shi PY. Zhang X, et al. Emerg Microbes Infect. 2019;8(1):585-602. doi: 10.1080/22221751.2019.1598291. Emerg Microbes Infect. 2019. PMID: 30958095 Free PMC article. - Zika Virus Proteins NS2A and NS4A Are Major Antagonists that Reduce IFN-β Promoter Activity Induced by the MDA5/RIG-I Signaling Pathway.
Ngueyen TTN, Kim SJ, Lee JY, Myoung J. Ngueyen TTN, et al. J Microbiol Biotechnol. 2019 Oct 28;29(10):1665-1674. doi: 10.4014/jmb.1909.09017. J Microbiol Biotechnol. 2019. PMID: 31581385 - Zika infection and the development of neurological defects.
Russo FB, Jungmann P, Beltrão-Braga PCB. Russo FB, et al. Cell Microbiol. 2017 Jun;19(6). doi: 10.1111/cmi.12744. Epub 2017 May 3. Cell Microbiol. 2017. PMID: 28370966 Review. - Vav Proteins in Development of the Brain: A Potential Relationship to the Pathogenesis of Congenital Zika Syndrome?
Norbury AJ, Jolly LA, Kris LP, Carr JM. Norbury AJ, et al. Viruses. 2022 Feb 14;14(2):386. doi: 10.3390/v14020386. Viruses. 2022. PMID: 35215978 Free PMC article. Review.
Cited by
- Human organoids: model systems for human biology and medicine.
Kim J, Koo BK, Knoblich JA. Kim J, et al. Nat Rev Mol Cell Biol. 2020 Oct;21(10):571-584. doi: 10.1038/s41580-020-0259-3. Epub 2020 Jul 7. Nat Rev Mol Cell Biol. 2020. PMID: 32636524 Free PMC article. Review. - Artificial Selection Finds New Hypotheses for the Mechanism of _Wolbachia-_Mediated Dengue Blocking in Mosquitoes.
Ford SA, Albert I, Allen SL, Chenoweth SF, Jones M, Koh C, Sebastian A, Sigle LT, McGraw EA. Ford SA, et al. Front Microbiol. 2020 Jul 7;11:1456. doi: 10.3389/fmicb.2020.01456. eCollection 2020. Front Microbiol. 2020. PMID: 32733407 Free PMC article. - Let's Get Physical: Flavivirus-Host Protein-Protein Interactions in Replication and Pathogenesis.
Fishburn AT, Pham OH, Kenaston MW, Beesabathuni NS, Shah PS. Fishburn AT, et al. Front Microbiol. 2022 Mar 3;13:847588. doi: 10.3389/fmicb.2022.847588. eCollection 2022. Front Microbiol. 2022. PMID: 35308381 Free PMC article. Review. - Modeling Host-Virus Interactions in Viral Infectious Diseases Using Stem-Cell-Derived Systems and CRISPR/Cas9 Technology.
Kim J, Koo BK, Yoon KJ. Kim J, et al. Viruses. 2019 Jan 30;11(2):124. doi: 10.3390/v11020124. Viruses. 2019. PMID: 30704043 Free PMC article. Review. - Generation of human brain region-specific organoids using a miniaturized spinning bioreactor.
Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. Qian X, et al. Nat Protoc. 2018 Mar;13(3):565-580. doi: 10.1038/nprot.2017.152. Epub 2018 Feb 22. Nat Protoc. 2018. PMID: 29470464 Free PMC article.
References
- Besnard M, Eyrolle-Guignot D, Guillemette-Artur P, Lastere S, Bost-Bezeaud F, Marcelis L, Abadie V, Garel C, Moutard ML, Jouannic JM, et al. Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill. 2016:21. - PubMed
- Buchman JJ, Tsai LH. Spindle regulation in neural precursors of flies and mammals. Nat Rev Neurosci. 2007;8:89–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM111514/GM/NIGMS NIH HHS/United States
- P01 NS097206/NS/NINDS NIH HHS/United States
- R35 NS097370/NS/NINDS NIH HHS/United States
- T32 GM007814/GM/NIGMS NIH HHS/United States
- R21 MH087874/MH/NIMH NIH HHS/United States
- T32 GM007309/GM/NIGMS NIH HHS/United States
- T32 GM007445/GM/NIGMS NIH HHS/United States
- R33 MH087874/MH/NIMH NIH HHS/United States
- R37 NS047344/NS/NINDS NIH HHS/United States
- U19 MH106434/MH/NIMH NIH HHS/United States
- R25 NS065729/NS/NINDS NIH HHS/United States
- K12 NS098482/NS/NINDS NIH HHS/United States
- R21 ES021957/ES/NIEHS NIH HHS/United States
- R21 NS095348/NS/NINDS NIH HHS/United States
- R21 AI119530/AI/NIAID NIH HHS/United States
- R56 NS047344/NS/NINDS NIH HHS/United States
- R01 NS048271/NS/NINDS NIH HHS/United States
- R01 NS051630/NS/NINDS NIH HHS/United States
- U19 AI131130/AI/NIAID NIH HHS/United States
- R01 MH105128/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous