Platelets reduce anoikis and promote metastasis by activating YAP1 signaling - PubMed (original) (raw)
doi: 10.1038/s41467-017-00411-z.
Morgan L Taylor 1, Tony Gutschner 3 4, Sunila Pradeep 1, Min Soon Cho 5, Jianting Sheng 6, Yasmin M Lyons 1, Archana S Nagaraja 1, Robert L Dood 1, Yunfei Wen 1, Lingegowda S Mangala 1 7, Jean M Hansen 1, Rajesha Rupaimoole 1, Kshipra M Gharpure 1, Cristian Rodriguez-Aguayo 7 8, Sun Young Yim 9, Ju-Seog Lee 9, Cristina Ivan 8, Wei Hu 1, Gabriel Lopez-Berestein 7 8, Stephen T Wong 6, Beth Y Karlan 10, Douglas A Levine 11, Jinsong Liu 12, Vahid Afshar-Kharghan 13, Anil K Sood 14 15 16
Affiliations
- PMID: 28827520
- PMCID: PMC5566477
- DOI: 10.1038/s41467-017-00411-z
Platelets reduce anoikis and promote metastasis by activating YAP1 signaling
Monika Haemmerle et al. Nat Commun. 2017.
Abstract
Thrombocytosis is present in more than 30% of patients with solid malignancies and correlates with worsened patient survival. Tumor cell interaction with various cellular components of the tumor microenvironment including platelets is crucial for tumor growth and metastasis. Although it is known that platelets can infiltrate into tumor tissue, secrete pro-angiogenic and pro-tumorigenic factors and thereby increase tumor growth, the precise molecular interactions between platelets and metastatic cancer cells are not well understood. Here we demonstrate that platelets induce resistance to anoikis in vitro and are critical for metastasis in vivo. We further show that platelets activate RhoA-MYPT1-PP1-mediated YAP1 dephosphorylation and promote its nuclear translocation which induces a pro-survival gene expression signature and inhibits apoptosis. Reduction of YAP1 in cancer cells in vivo protects against thrombocytosis-induced increase in metastasis. Collectively, our results indicate that cancer cells depend on platelets to avoid anoikis and succeed in the metastatic process.Platelets have been associated with increased tumor growth and metastasis but the mechanistic details of this interaction are still unclear. Here the authors show that platelets improve anoikis resistance of cancer cells and increase metastasis by activating Yap through a RhoA/MYPT-PP1 pathway.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Fig. 1
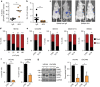
Platelets facilitate metastasis in vivo and reduce anoikis. a Plot showing percent of SYTOX Red positive EPCAM + MDAH-2774 tumor cells in ascites after treatment with control IgG or anti-platelet antibody (APA, n = 5, two-sided Student’s _t_-test). b, c Number of liver nodules b and representative bioluminescence imaging pictures c 5 weeks after intrasplenic injection of 2 × 106 SW620 colon cancer cells (n = 10, two-sided Student’s _t_-test). d Number of dead (SYTOX Red positive, black) and living (SYTOX Red negative, red) HEYA8 cells after 72 hours of low attachment and/or co-incubation with increasing numbers of platelets (n = 3, two-sided Student’s _t_-test). e Number of dead (SYTOX\ Red positive, black) and living (SYTOX Red negative, red) OVCAR8, SKOV3, MDAH-2774 and SW620 cells after 72 h of low attachment and/or co-incubation with 100 × 106 platelets (n = 3, two-sided Student’s _t_-test). f Percentage of HEYA8 or OVCAR8 human ovarian cancer cells in sub-G1 phase of the cell cycle after 72 h under low-attachment conditions (n = 3, two-sided Student’s _t_-test). g Protein analysis and quantification of full length and cleaved PARP in HEYA8 and OVCAR8 cells after 72 h under low-attachment conditions (short exposure of PARP upper panel, long exposure of PARP lower panel, n = 3, two-sided Student’s _t_-test). GAPDH was used as a loading control. Bars and error bars represent mean values and the corresponding SEMs (*p < 0.05, **p < 0.01, ***p < 0.001)
Fig. 2
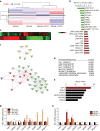
Platelets induce a YAP1-specific gene signature in cancer cells. a Heat map showing differentially regulated proteins as analyzed by reverse phase protein array (RPPA) in HEYA8 and HEYA8 co-incubated with platelets for two hours (n = 2). b Top 10 down- and upregulated proteins (+plts vs. control) after RPPA analysis. c Heat map depicting differentially regulated genes in HEYA8 or HEYA8 cells co-incubated with 100 × 106 platelets under low-attachment conditions for 24 hours (n = 3, p < 0.001). d Enriched pathways with indicated _p_-values of upregulated genes in platelet co-incubated HEYA8 cells using Gene Set Enrichment Analysis (GSEA,
). e Upstream analysis of gene expression changes in HEYA8 ovarian cancer cells co-incubated with platelets using Ingenuity Pathway Analysis (IPA,
http://www.ingenuity.com/products/ipa
). f Random walk analysis highlighting YAP1 protein as the main connector to transcriptional changes in HEYA8 co-incubated with platelets. g QRT–PCR analysis of YAP1, NDRG1, DDIT4, CDC20, CTGF, MCM5, MCM6, and MCM10 in HEYA8 cells after 24 and 48 h of co-incubation with platelets. 18S was used as the housekeeping gene (n = 3, two-sided Student’s _t_-test). h QRT–PCR analysis of YAP1, NDRG1, DDIT4, CDC20, CTGF, MCM5, MCM6, and MCM10 after YAP1 knockdown with two different siRNAs (72 hours after transfection, n = 3). 18S was used as the housekeeping gene (n = 3, two-sided Student’s _t_-test). Bars and error bars represent mean values and the corresponding SEMs (*p < 0.05, **p < 0.01, ***p < 0.001)
Fig. 3
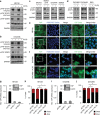
YAP1 is activated by platelets and is indispensable for platelet-induced anoikis resistance. a, b Western blot analysis of phosphorylated YAP1 (S127 and S397) and total YAP1 in HEYA8 a and OVCAR8 b cells after two hours under low-attachment conditions with or without platelet co-incubation. GAPDH was used as a loading control (n = 5). c, d Western blot analysis of phosphorylated YAP1 (S127) and total YAP1 in SKOV3, MDAH-2774, OVCAR4, SW620 c and OVCAR5, OVCA432 and RKO d GAPDH was used as a loading control (n = 3). e, f Immunofluorescence staining of YAP1 in HEYA8 e and OVCAR8 f cells after two hours under low-attachment conditions with (lower panels) or without (upper panels) platelet co-incubation. Inlets showing higher magnification of cells on the right side of the panels. Nuclear counterstain was done using Hoechst 33342 (n = 3). Scale bars = 20 µm. g QRT–PCR and western blot analysis in HEYA8 cells showing efficiency of YAP1 knockdown on the RNA and protein level using two different siRNAs (n = 3). h Bar graphs showing number of dead (SYTOX Red positive, black) and living (SYTOX Red negative, red) HEYA8 cells after 72 h of low attachment and 96 h after siRNA transfection (n = 3, two-sided Student’s _t_-test). i QRT–PCR and western blot analysis in OVCAR8 cells showing efficiency of YAP1 knockdown on the RNA and protein level using two different siRNAs (n = 3). j Bar graphs showing number of dead (SYTOX Red positive, black) and living (SYTOX Red negative, red) OVCAR8 cells after 72 h of low attachment and 96 h after siRNA transfection (n = 3, two-sided Student’s _t_-test). Bars and error bars represent mean values and the corresponding SEMs (*p < 0.05, **p < 0.01, ***p < 0.001)
Fig. 4
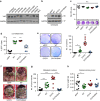
YAP1 overexpression increases anoikis resistance and metastasis. a Analysis of YAP1 protein expression in ovarian and colon cancer cell lines. GAPDH was used as a loading control (n = 2). b Validation of overexpression of YAP1 with wild-type (WT) YAP1, constitutively active (S127A) YAP1 and TEAD-defective mutant (S127A/S94A) YAP1 in OVCA432 human ovarian cancer cells. GAPDH was used as a loading control (n = 3). c Cell viability analysis (upper panel) and representative crystal violet staining (lower panel) of OVCA432 cells transduced with control or YAP1 overexpressing constructs 72 h after seeding into 96-well plates (n = 6, one-way ANOVA followed by a Tukey’s multiple comparison post hoc test). d Analysis of GFP-positive, viable (SYTOX Red negative) cells after 72 h of low attachment in OVCA432 cells transduced with control or YAP1 overexpressing constructs (n = 6, one-way ANOVA followed by a Tukey’s multiple comparison post hoc test). e Anchorage-independent growth assay in soft agar using OVCA432 transduced with control or YAP1 overexpressing constructs and quantification of colonies after seeding 15,000 cells and incubation for 14 days (n = 3, one-way ANOVA followed by a Tukey’s multiple comparison post-hoc test). f–h Representative necropsy pictures f, number of metastatic nodules g and weight of ovarian primary tumor h after intraovarian injection of OVCA432 overexpressed with either eGFP-control, YAPWT, YAPS127A or YAPS127A/S94A constructs (n = 10, one-way ANOVA followed by a Tukey’s multiple comparison post-hoc test). Bars and error bars represent mean values and the corresponding SEMs (*p < 0.05, **p < 0.01, ***p < 0.001)
Fig. 5
Inhibition of YAP1 in vivo impedes thrombocytosis-enhanced metastasis. a, b Immunohistochemical staining and quantification of nuclear versus cytoplasmic YAP1 protein expression in 21 matched primary high-grade serous ovarian carcinomas and metastatic nodules. N > C: nuclear YAP1 > cytoplasmic YAP1; N = C: nuclear YAP1 = cytoplasmic YAP1; N < C: nuclear YAP1 < cytoplasmic YAP1 (_n_ = 21, two-sided Student’s _t_-test). **c**–**e** Aggregate tumor weight of ovarian primary tumor **c**, number of metastatic tumor nodules **d** and representative necropsy pictures **e** in mice receiving control (siCTRL) or YAP1 (siYAP) siRNA with or without platelet transfusion (PLTS TRANS) twice weekly, respectively (_n_ = 10, one-way ANOVA followed by a Tukey’s multiple comparison post hoc test). **f**, **g** Validation of YAP1 knockdown using western blot analysis of whole tumor lysates **f** or immunohistochemical staining **g** with antibodies against YAP1 (for WB and IHC) and phosphorylated YAP1 (S127, for WB). GAPDH was used as a loading control (for WB). Representative immunohistochemical images and western blot after processing of tumors from 7 mice. **h** Immunohistochemical analysis and quantification of nuclear versus cytoplasmic YAP1 positive tumor cells in tumor sections of primary and metastatic nodules. N > C: nuclear YAP1 > cytoplasmic YAP1; N = C: nuclear YAP1 = cytoplasmic YAP1; N < C: nuclear YAP1 < cytoplasmic YAP1 (n = 7, two-sided Student’s _t_-test). Representative immunohistochemical images after processing of primary and metastatic nodules from 7 mice. i Immunohistochemical staining for cleaved caspase 3 (CC3) and quantification of the number of CC3-positive cells per high power field (HPF) in tumors treated with control (siCTRL) or YAP1 (siYAP) siRNA with or without platelet transfusions twice weekly (n = 7, one-way ANOVA followed by a Tukey’s multiple comparison post-hoc test). Representative immunohistochemical images after processing of tumors from seven mice. Bars and error bars represent mean values and the corresponding SEMs (*p < 0.05, **p < 0.01, ***p < 0.001). Scale bars = 50 µm
Fig. 6
Regulation of YAP1 activity by RhoA-MYPT1-PP1 axis controls anoikis. a Analysis of active RhoA using RhoA-GTP pulldown assay in HEYA8 and OVCAR8 cells with or without platelet co-incubation for two hours. RhoA expression in input samples was used as control for quantification and GAPDH as loading control (n = 2, two-sided Student’s _t_-test). b Protein analysis of phosphorylated (S127) and total YAP1 in HEYA8 and OVCAR8 cells after platelet co-incubation for two hours with or without 1.0 µg ml−1 of Rho inhibitor C3 transferase (n = 3). c Bar graphs showing the number of dead (SYTOX Red positive, black) and living (SYTOX Red negative, red) HEYA8 or OVCAR8 ovarian cancer cells after growing for 72 hours in low attachment with or without platelet co-incubation and treatment with 1.0 µg ml−1 of Rho inhibitor C3 transferase (n = 3, two-sided Student’s _t_-test). d Protein analysis for phosphorylated MYPT1 (T853, T696 and S507), total MYPT1, phosphorylated YAP1 (S127) and total YAP1 in HEYA8 and OVCAR8 ovarian cancer cells after two hours of low attachment with platelet co-incubation or treatment of 1.0 U ml−1 Rho activator (n = 3). e YAP1 co-immunoprecipitation assays showing MYPT1 interaction with YAP1 after platelet co-incubation or treatment with 1.0 U ml−1 Rho activator for two hours under low-attachment conditions. Similar pulldown of YAP1 protein was confirmed. GAPDH was used as loading control (n = 3). f, g Protein analysis for phosphorylated (S127) and total YAP1 after platelet co-incubation for two hours with or without pre-treatment with 30 nM phosphatase inhibitor Calyculin A (CA) for 30 minutes in HEYA8 f and OVCAR8 g cells (left panels, n = 3). Bar graphs representing the number of dead (SYTOX Red positive, black) and living (SYTOX Red negative, red) HEYA8 f or OVCAR8 g ovarian cancer cells after platelet co-incubation for 72 h with or without pre-treatment with 30 nM phosphatase inhibitor Calyculin A (CA) for 30 min (right panels, n = 3, two-sided Student’s _t_-test). h Proposed model of YAP1 activation, anoikis resistance and metastasis in cancer cells by platelets
Similar articles
- MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer.
Yu SJ, Hu JY, Kuang XY, Luo JM, Hou YF, Di GH, Wu J, Shen ZZ, Song HY, Shao ZM. Yu SJ, et al. Clin Cancer Res. 2013 Mar 15;19(6):1389-99. doi: 10.1158/1078-0432.CCR-12-1959. Epub 2013 Jan 22. Clin Cancer Res. 2013. PMID: 23340296 - PKCι regulates nuclear YAP1 localization and ovarian cancer tumorigenesis.
Wang Y, Justilien V, Brennan KI, Jamieson L, Murray NR, Fields AP. Wang Y, et al. Oncogene. 2017 Jan 26;36(4):534-545. doi: 10.1038/onc.2016.224. Epub 2016 Jun 20. Oncogene. 2017. PMID: 27321186 Free PMC article. - FGF8 promotes colorectal cancer growth and metastasis by activating YAP1.
Liu R, Huang S, Lei Y, Zhang T, Wang K, Liu B, Nice EC, Xiang R, Xie K, Li J, Huang C. Liu R, et al. Oncotarget. 2015 Jan 20;6(2):935-52. doi: 10.18632/oncotarget.2822. Oncotarget. 2015. PMID: 25473897 Free PMC article. - Understanding the cell survival mechanism of anoikis-resistant cancer cells during different steps of metastasis.
Khan SU, Fatima K, Malik F. Khan SU, et al. Clin Exp Metastasis. 2022 Oct;39(5):715-726. doi: 10.1007/s10585-022-10172-9. Epub 2022 Jul 13. Clin Exp Metastasis. 2022. PMID: 35829806 Review. - Platelet-mediated tumor metastasis mechanism and the role of cell adhesion molecules.
Liu Y, Zhang Y, Ding Y, Zhuang R. Liu Y, et al. Crit Rev Oncol Hematol. 2021 Nov;167:103502. doi: 10.1016/j.critrevonc.2021.103502. Epub 2021 Oct 15. Crit Rev Oncol Hematol. 2021. PMID: 34662726 Review.
Cited by
- LMP1 promotes nasopharyngeal carcinoma metastasis through NTRK2-mediated anoikis resistance.
Li Z, Zhou Z, Wu X, Zhou Q, Liao C, Liu Y, Li D, Shen L, Feng D, Yang L. Li Z, et al. Am J Cancer Res. 2020 Jul 1;10(7):2083-2099. eCollection 2020. Am J Cancer Res. 2020. PMID: 32775002 Free PMC article. - Proteomic and transcriptomic profiling identifies mediators of anchorage-independent growth and roles of inhibitor of differentiation proteins in invasive lobular carcinoma.
Tasdemir N, Ding K, Savariau L, Levine KM, Du T, Elangovan A, Bossart EA, Lee AV, Davidson NE, Oesterreich S. Tasdemir N, et al. Sci Rep. 2020 Jul 13;10(1):11487. doi: 10.1038/s41598-020-68141-9. Sci Rep. 2020. PMID: 32661241 Free PMC article. - YAP Enhances Tumor Cell Dissemination by Promoting Intravascular Motility and Reentry into Systemic Circulation.
Benjamin DC, Kang JH, Hamza B, King EM, Lamar JM, Manalis SR, Hynes RO. Benjamin DC, et al. Cancer Res. 2020 Sep 15;80(18):3867-3879. doi: 10.1158/0008-5472.CAN-20-0212. Epub 2020 Jun 26. Cancer Res. 2020. PMID: 32591412 Free PMC article. - Tumor microenvironment in ovarian cancer peritoneal metastasis.
Mei S, Chen X, Wang K, Chen Y. Mei S, et al. Cancer Cell Int. 2023 Jan 25;23(1):11. doi: 10.1186/s12935-023-02854-5. Cancer Cell Int. 2023. PMID: 36698173 Free PMC article. Review. - Tumor-infiltrating platelets predict postoperative recurrence and survival in resectable pancreatic neuroendocrine tumor.
Xu SS, Xu HX, Wang WQ, Li S, Li H, Li TJ, Zhang WH, Liu L, Yu XJ. Xu SS, et al. World J Gastroenterol. 2019 Nov 7;25(41):6248-6257. doi: 10.3748/wjg.v25.i41.6248. World J Gastroenterol. 2019. PMID: 31749595 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- UH2TR000943/NH/NIH HHS/United States
- T32 CA101642/CA/NCI NIH HHS/United States
- CA016672/NH/NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- P50 CA083639/NH/NIH HHS/United States
- R01 CA177909/CA/NCI NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases