The senescent bystander effect is caused by ROS-activated NF-κB signalling - PubMed (original) (raw)
The senescent bystander effect is caused by ROS-activated NF-κB signalling
Glyn Nelson et al. Mech Ageing Dev. 2018 Mar.
Abstract
Cell senescence is an important driver of the ageing process. The accumulation of senescent cells in tissues is accelerated by stress signals from senescent cells that induce DNA damage and ultimately senescence in bystander cells. We examine here the interplay of senescence-associated mitochondrial dysfunction (SAMD)-driven production of reactive oxygen species (ROS) and senescence-associated secretory phenotype (SASP) in causing the bystander effect. We show that in various modes of fibroblast senescence ROS are necessary and sufficient to activate the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which facilitates a large part of the SASP. This ROS-NF-κB axis causes the DNA damage response in bystander cells. Cytokines IL-6 and IL-8 are major components of the pro-inflammatory SASP in senescent fibroblasts. However, their activation in senescence is only partially controlled by NF-κB, and they are thus not strong candidates as intercellular mediators of the bystander effect as mediated by the ROS-NF-κB axis.
Keywords: Bystander; Mitochondria; NF-κB; Reactive oxygen; Senescence.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
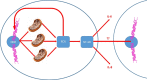
Graphical abstract
Fig. 1
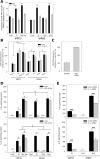
ROS controls SASP, but SASP does not control SAMD. A: ROS release from senescent cells is necessary for activation of NF-κB. Nuclear and cytosolic localization of the NF-κB transcription factor, RelA, was measured at single cell level by immunofluorescence. Frequencies of cells with nuclear: cytosolic NF-κB signal ratio >1 are shown. Cells were untreated (Ctrl) or treated with catalase (100 U/ml for 2 days prior to fixation, +Cat). Experiments were performed three times, data shown are means +/− SD. Statistical tests were performed as one way ANOVA with a Dunn's post-hoc test. B: NF-κB activity does not impact on senescence-associated ROS generation. Cultured cells were grown with and without NF-κB inhibitor (Bay11-7082, 1 μM) for 2 days prior to measuring DHE fluorescence by flow cytometry. All modes of senescence increased fluorescence intensity, but there were no differences between cells treated with the NF-κB inhibitor compared to their respective controls. Each experiment was performed three times, data shown are means +/− SD. Statistical tests were performed as one way ANOVA with a Dunn's post-hoc test. C: Transgenic inhibition of NF-κB signalling does not affect ROS production in replicative senescence. MRC5 transduced with a lentiviral dominant negative IκBα −IRES-EGFP construct were analysed as actively proliferating as well as grown to replicative senescence for DHE fluorescence by flow cytometry. Each experiment was performed three times, data shown are means +/− SD. Statistical tests were performed as a Student's T-test (proliferating control versus Rep Sen), and also as a one way ANOVA with parental MRC5 cells (shown in B), which showed no significant difference between the two replicatively senescent populations. D: SASP cytokines are partially controlled by NF-κB in replicative and stress-induced senescence. ELISA measurements of IL-6 (top) and IL-8 (bottom) in medium from cells as indicated treated with and without NF-κB inhibitor (Bay11-7082, 1 μM). Experiments were performed twice (duplicate cultures ran in triplicate), data plotted as mean +/− SD. Statistical tests were performed as two way ANOVA with Holm-Sidak post-hoc tests. E: IL-1α controls SASP cytokines in two senescence modes. ELISA measurements of IL-6 (top) and IL-8 (bottom) in medium of cells 3 days after transfection with IL-1α siRNA or scrambled control siRNA. Experiments were performed twice (duplicate cultures ran in triplicate), data plotted as mean +/− SD. Statistical tests were performed using a one way ANOVA with Holm-Sidak post-hoc tests.
Fig. 2
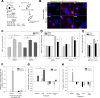
Role of SASP and SAMD for the bystander effect. A: Cartoon model representing the in vitro model system designed to analyse the senescent bystander effect. Three different modes of cellular senescence were employed in the inducer cells: replicative senescence in MRC5 (Rep Sen), oncogene induced senescence via RAS in IMR90 (OIS), and X-ray irradiation induced senescence (10 days post 20 Gy) of either MRC5 or IMR90 (IR). In each case, young proliferating MRC5 cells stably expressing mcherry-53BP1 were used as bystander cells, cultured in a 1:1 ratio, and mcherry-53BP1 foci were counted in bystander cells to determine the level of DNA damage. B: Representative images of co-cultured bystander cells (mCherry-53BP1 expressing MRC5) in the presence of inducer cells (MRC5 top, IMR90 bottom). The mode of senescence for each inducer type is shown in the image overlays. Dashed outlines highlight inducer cell nuclei. Blue represents DAPI staining, red mCherry-53BP1. Note cytosolic autofluorescence present in senescent inducers. Scale bar = 10 μm. C: All modes of senescence induce a bystander response. Co-cultures were grown for 7 days with either young proliferating control cells (Prolif.) or inducer cells in the indicated senescence mode. 53BP1 foci frequencies in the bystander cells are shown. Significant differences (* P < 0.05) in comparison to respective controls are indicated, Kruskal-Wallis ANOVA on Ranks with Holm-Sidak post hoc test for pairwise comparison between groups. Neither cell strain nor senescence mode had a differential impact on the bystander effect (Two-way ANOVA test). Data plotted are mean +/− SD from 100 to 150 cells per group from 3 independent replicates. D, E: ROS and NF-κB contribute to the bystander response. Proliferating control (Prolif or −RAS) or senescent inducer cells as indicated were co-cultured with proliferating MRC5:53BP1 bystander cells in the presence of either Catalase (Antiox., 100U/ml), NF-κB inhibitor Bay11-7082 (1 μM) or carrier (DMSO). Cultures were co-cultured for either 6 days (D) or 21 days (E). 53BP1 foci frequencies in the bystander cells are shown. CAT significantly inhibited the bystander effect in all experiments, while Bay11-7082 reduced the bystander effect from MRC5-RS only after prolonged co-culture. Data plotted are mean +/− SD from 180 to 250 cells per group from 2 independent replicates. Kruskal-Wallis ANOVA on Ranks with Holm-Sidak post hoc test for pairwise comparison between groups, asterisks show P < 0.05. F: ΔIκBα expression inhibits the bystander effect from replicatively senescent MRC5. Young (Prolif) or replicatively senescent (RS) MRC5 fibroblast stably transduced with either mCherry or ΔIκBα we co-cultured with MRC5:53BP1 bystander cells. Differences between 53BP1 foci frequencies in bystander cells to those in proliferating cells alone are shown. Experiments were performed in triplicate and between 60 and 120 cells were analysed per treatment and replicate. Data are mean ± SE (n = 3-5), with approximately 100 cells per experiment. G: TNFα pre-stimulation enhances the bystander effect, and CAT abrogates it. Senescent inducer cells were stimulated with TNFα (TNF) or not (UNSTIM) before co-culture with reporter cells for 21 days. CAT (ANTIOX) was added during co-culture. Differences between 53BP1 foci frequencies in bystander cells to those in proliferating cells alone are shown. Data are mean ± SE (n = 3-5), with approximately 100 cells per experiment. H: Inhibitors of NF-κB, mTORC1 and p38MAPK equally suppress the bystander effect. Replicatively senescent MRC5 fibroblasts were co-cultured with reporter cells for 21 days with either DMSO as carrier, Bay11-7082 (BAY), the mTORC1 inhibitor torin1 (TOR) or the p38MAPK inhibitor UR-13756. Differences between 53BP1 foci frequencies in bystander cells to those in proliferating cells alone are shown. Data are mean ± SE (n = 3-5), with approximately 100 cells per experiment. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Similar articles
- IL1- and TGFβ-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine 'bystander senescence'.
Hubackova S, Krejcikova K, Bartek J, Hodny Z. Hubackova S, et al. Aging (Albany NY). 2012 Dec;4(12):932-51. doi: 10.18632/aging.100520. Aging (Albany NY). 2012. PMID: 23385065 Free PMC article. - Role of Clusterin/NF-κB in the secretion of senescence-associated secretory phenotype in Cr(VI)-induced premature senescent L-02 hepatocytes.
Liang Y, Liang N, Ma Y, Tang S, Ye S, Xiao F. Liang Y, et al. Ecotoxicol Environ Saf. 2021 Aug;219:112343. doi: 10.1016/j.ecoenv.2021.112343. Epub 2021 May 18. Ecotoxicol Environ Saf. 2021. PMID: 34020271 - The canonical NF-κB pathway differentially protects normal and human tumor cells from ROS-induced DNA damage.
Sfikas A, Batsi C, Tselikou E, Vartholomatos G, Monokrousos N, Pappas P, Christoforidis S, Tzavaras T, Kanavaros P, Gorgoulis VG, Marcu KB, Kolettas E. Sfikas A, et al. Cell Signal. 2012 Nov;24(11):2007-23. doi: 10.1016/j.cellsig.2012.06.010. Epub 2012 Jun 29. Cell Signal. 2012. PMID: 22750558 Free PMC article. - Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP).
Salminen A, Kauppinen A, Kaarniranta K. Salminen A, et al. Cell Signal. 2012 Apr;24(4):835-45. doi: 10.1016/j.cellsig.2011.12.006. Epub 2011 Dec 11. Cell Signal. 2012. PMID: 22182507 Review. - Cellular senescence: Molecular mechanisms and pathogenicity.
Wei W, Ji S. Wei W, et al. J Cell Physiol. 2018 Dec;233(12):9121-9135. doi: 10.1002/jcp.26956. Epub 2018 Aug 5. J Cell Physiol. 2018. PMID: 30078211 Review.
Cited by
- Fisetin reduces the senescent tubular epithelial cell burden and also inhibits proliferative fibroblasts in murine lupus nephritis.
Ijima S, Saito Y, Nagaoka K, Yamamoto S, Sato T, Miura N, Iwamoto T, Miyajima M, Chikenji TS. Ijima S, et al. Front Immunol. 2022 Nov 17;13:960601. doi: 10.3389/fimmu.2022.960601. eCollection 2022. Front Immunol. 2022. PMID: 36466895 Free PMC article. - Inhibitory immune checkpoints suppress the surveillance of senescent cells promoting their accumulation with aging and in age-related diseases.
Salminen A. Salminen A. Biogerontology. 2024 Oct;25(5):749-773. doi: 10.1007/s10522-024-10114-w. Epub 2024 Jul 1. Biogerontology. 2024. PMID: 38954358 Free PMC article. Review. - Senolytic Therapy: A Potential Approach for the Elimination of Oncogene-Induced Senescent HPV-Positive Cells.
Saleh T, Khasawneh AI, Himsawi N, Abu-Raideh J, Ejeilat V, Elshazly AM, Gewirtz DA. Saleh T, et al. Int J Mol Sci. 2022 Dec 8;23(24):15512. doi: 10.3390/ijms232415512. Int J Mol Sci. 2022. PMID: 36555154 Free PMC article. Review. - The bystander effect contributes to the accumulation of senescent cells in vivo.
da Silva PFL, Ogrodnik M, Kucheryavenko O, Glibert J, Miwa S, Cameron K, Ishaq A, Saretzki G, Nagaraja-Grellscheid S, Nelson G, von Zglinicki T. da Silva PFL, et al. Aging Cell. 2019 Feb;18(1):e12848. doi: 10.1111/acel.12848. Epub 2018 Nov 21. Aging Cell. 2019. PMID: 30462359 Free PMC article. - TGF-β mediates aortic smooth muscle cell senescence in Marfan syndrome.
You W, Hong Y, He H, Huang X, Tao W, Liang X, Zhang Y, Li X. You W, et al. Aging (Albany NY). 2019 May 30;11(11):3574-3584. doi: 10.18632/aging.101998. Aging (Albany NY). 2019. PMID: 31147528 Free PMC article.
References
- Acosta J.C., O’Loghlen A., Banito A., Guijarro M.V., Augert A., Raguz S., Fumagalli M., Da Costa M., Brown C., Popov N., Takatsu Y., Melamed J., d’Adda di Fagagna F., Bernard D., Hernando E., Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. S0092-8674 (08)00619-3 [pii] - PubMed
- Acosta J.C., Banito A., Wuestefeld T., Georgilis A., Janich P., Morton J.P., Athineos D., Kang T.W., Lasitschka F., Andrulis M., Pascual G., Morris K.J., Khan S., Jin H., Dharmalingam G., Snijders A.P., Carroll T., Capper D., Pritchard C., Inman G.J., Longerich T., Sansom O.J., Benitah S.A., Zender L., Gil J. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013 - PMC - PubMed
- Bagley M.C., Davis T., Rokicki M.J., Widdowson C.S., Kipling D. Synthesis of the highly selective p38 MAPK inhibitor UR-13756 for possible therapeutic use in Werner syndrome. Future Med. Chem. 2010;2:193–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/I020748/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/L016354/1/MRC_/Medical Research Council/United Kingdom
- 24009/CRUK_/Cancer Research UK/United Kingdom
- BB/K019260/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F010966/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources