Hydrogen-Rich Saline Alleviates Kidney Fibrosis Following AKI and Retains Klotho Expression - PubMed (original) (raw)
Hydrogen-Rich Saline Alleviates Kidney Fibrosis Following AKI and Retains Klotho Expression
Jing Chen et al. Front Pharmacol. 2017.
Abstract
Purpose: Acute kidney injury (AKI) is a prominent risk factor for the development of chronic kidney disease (CKD). To date, the related mechanism and effective therapy have not been rigorously explored. The present study aims to investigate the reno-protection of hydrogen-rich saline (HRS) against ischemia/reperfusion (IR)-induced AKI. Methods: Adult male C57 mice were randomly allocated into three groups: Sham, IR, IR+HRS. Renal IR injury model was generated via 35 min occlusion of bilateral kidney pedicles, and then, mice were administered with different treatments intraperitoneally in various groups. After 14- or 28-day treatment, mice were perfused and the kidneys were collected following reperfusion. Many proteins were detected by western blots, including renal fibrotic proteins [a-smooth muscle actin (a-SMA), collagen I (Col I)], Klotho, the methylation of Klotho, damage-regulated autophagy modulator (Beclin-1), and microtubule-associated protein light 3-II (LC3-II). Finally, the levels of serum blood urea nitrogen (BUN) and creatinine (Cr) were measured to investigate the renal function. Results: Histological data showed that the HRS treatment significantly decreased the fibrosis in renal tissues when compared with the IR group, and both of BUN and Cr were lower in the HRS group than IR group (8.9 ± 0.6 vs. 9.9 ± 0.1 mmol/l, 51 ± 6.5 vs. 60 ± 5.8 μmol/l) (P < 0.05). The expression of fibrotic markers, a-SMA and Col I, showed a robust increase in IR injury models than the Sham group, which was consistent with the result of Trichrome staining. However, the levels of a-SMA and Col I expression were sharply decreased in the IR+HRS group (P < 0.05). IR injury also enhanced LC3-II and Beclin-1 expression, but decreased Klotho level. The Klotho level was alleviated by HRS, but LC3-II and Beclin-1 were starkly enhanced in HRS group (P < 0.05). Conclusion: HRS showed a protective effect in the prevention of renal injury and could inhibit renal fibrosis after IR injury in mice. This role of HRS might be exerted via retaining Klotho expression and activating autophagy in the kidney.
Keywords: Klotho; acute kidney injury; fibrosis; hydrogen.
Figures
FIGURE 1
HRS Reduces the Histopathologic Damage in Mice with IR injury. (A) Representative photomicrographs of renal tissues submitted to hematoxylin-eosin and Masson Trichrome staining (200× magnification). Renal fibrosis scores provided semi-quantitative data of Trichrome staining findings. (B,C) Changes of fibrotic a-smooth muscle actin (a-SMA) and collagen I (Col I) levels by immunoblot were consistent with Trichrome staining data, with robustly increased amounts in IR models. ∗P < 0.05, ∗∗P < 0.01.
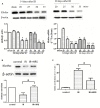
FIGURE 2
HRS unregulated Klotho expression and methylation level. (A) Renal Klotho levels at 14 and 28 days after IR in 20/25/30/35-min ischemia models. The protein level of renal Klotho was decreased at about 14 days after IR in 30-min ischemia models, with subsequent decrease until 28 days after the injury (∗P < 0.05). The gene expression of Klotho in 20-min ischemia models was significantly higher than the normal condition of the sham group at 14 days after IR, and this increase was abolished at 28 days after the injury (A) (compared with sham group, ∗P < 0.05). Both 30- and 35-min groups showed decreased Klotho gene expression at 14 days (compared with sham group, ∗∗P < 0.01). At 28 days, Klotho gene expression showed a decreasing trend in 30-min models whereas it remained at a lower level in 35-min models (compared with sham group, ∗∗P < 0.01). (B) The expression of renal Klotho was sharply increased in HRS group (compared with IR group, ∗P < 0.05). (C) The methylation level of Klotho in HRS group was decreased (compared with IR group, ∗P < 0.05).
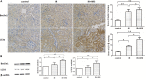
FIGURE 3
The expression of autophagy markers in these groups. (A,B) HRS enhanced the protein levels of autophagy markers in renal tissues of IR mice 28 days after reperfusion. Renal samples were collected 28 days after reperfusion to measure Beclin-1 and LC3-II expression levels by immunohistochemistry and western blot. LC3-II and Beclin-1 in the IR group were concurrently increased, compared to the sham group (∗P < 0.05). HRS treatment significantly elevated LC3-II and Beclin-1 expression levels compared with the IR group (∗P < 0.05).
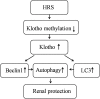
FIGURE 4
Schematic illustration of the molecular mechanisms of HRS-induced renoprotection against AKI to CKD after IR.
Similar articles
- [Protective effects of Klotho protein on acute kidney injury in septic mice and its mechanism].
Sun M, Fan H, Le J, Chen G, Chen H, Li J, Zhu J. Sun M, et al. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019 Feb;31(2):160-164. doi: 10.3760/cma.j.issn.2095-4352.2019.02.008. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019. PMID: 30827302 Chinese. - Hydrogen-Rich Saline Attenuates Acute Kidney Injury After Liver Transplantation via Activating p53-Mediated Autophagy.
Du H, Sheng M, Wu L, Zhang Y, Shi D, Weng Y, Xu R, Yu W. Du H, et al. Transplantation. 2016 Mar;100(3):563-70. doi: 10.1097/TP.0000000000001052. Transplantation. 2016. PMID: 26714124 - The role of podocyte damage in the etiology of ischemia-reperfusion acute kidney injury and post-injury fibrosis.
Chen Y, Lin L, Tao X, Song Y, Cui J, Wan J. Chen Y, et al. BMC Nephrol. 2019 Mar 28;20(1):106. doi: 10.1186/s12882-019-1298-x. BMC Nephrol. 2019. PMID: 30922260 Free PMC article. - The protective mechanism of Klotho gene-modified bone marrow mesenchymal stem cells on acute kidney injury induced by rhabdomyolysis.
Ni W, Zhang Y, Yin Z. Ni W, et al. Regen Ther. 2021 Aug 13;18:255-267. doi: 10.1016/j.reth.2021.07.003. eCollection 2021 Dec. Regen Ther. 2021. PMID: 34466631 Free PMC article. Review. - Implications of Klotho Protein for Managing Kidney Disease - an Emerging Role in Therapeutics and Molecular Medicine.
Ray SK, Masarkar N, Mukherjee S. Ray SK, et al. Curr Mol Med. 2021;21(6):484-494. doi: 10.2174/1566524020666201120143313. Curr Mol Med. 2021. PMID: 33222666 Review.
Cited by
- Therapeutic effect of hydrogen and its mechanisms in kidney disease treatment.
Cheng J, Shi M, Sun X, Lu H. Cheng J, et al. Med Gas Res. 2024 Apr-Jun;14(2):48-53. doi: 10.4103/2045-9912.378880. Med Gas Res. 2024. PMID: 37929507 Free PMC article. Review. - Low-Flow Nasal Cannula Hydrogen Therapy.
Sano M, Shirakawa K, Katsumata Y, Ichihara G, Kobayashi E. Sano M, et al. J Clin Med Res. 2020 Oct;12(10):674-680. doi: 10.14740/jocmr4323. Epub 2020 Sep 21. J Clin Med Res. 2020. PMID: 33029275 Free PMC article. - Klotho as Potential Autophagy Regulator and Therapeutic Target.
Zhou H, Pu S, Zhou H, Guo Y. Zhou H, et al. Front Pharmacol. 2021 Oct 19;12:755366. doi: 10.3389/fphar.2021.755366. eCollection 2021. Front Pharmacol. 2021. PMID: 34737707 Free PMC article. Review. - Hydrogen: A Rising Star in Gas Medicine as a Mitochondria-Targeting Nutrient via Activating Keap1-Nrf2 Antioxidant System.
Cheng D, Long J, Zhao L, Liu J. Cheng D, et al. Antioxidants (Basel). 2023 Nov 30;12(12):2062. doi: 10.3390/antiox12122062. Antioxidants (Basel). 2023. PMID: 38136182 Free PMC article. Review. - Application of Electrolyzed Hydrogen Water for Management of Chronic Kidney Disease and Dialysis Treatment-Perspective View.
Nakayama M, Kabayama S, Miyazaki M. Nakayama M, et al. Antioxidants (Basel). 2024 Jan 11;13(1):90. doi: 10.3390/antiox13010090. Antioxidants (Basel). 2024. PMID: 38247514 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources