Spasmolytic Mechanism of Aqueous Licorice Extract on Oxytocin-Induced Uterine Contraction through Inhibiting the Phosphorylation of Heat Shock Protein 27 - PubMed (original) (raw)
Spasmolytic Mechanism of Aqueous Licorice Extract on Oxytocin-Induced Uterine Contraction through Inhibiting the Phosphorylation of Heat Shock Protein 27
Lu Yang et al. Molecules. 2017.
Abstract
Licorice derived from the roots and rhizomes of Glycyrrhiza uralensis Fisch. (Fabaceae), is one of the most widely-used traditional herbal medicines in China. It has been reported to possess significant analgesic activity for treating spastic pain. The aim of this study is to investigate the spasmolytic molecular mechanism of licorice on oxytocin-induced uterine contractions and predict the relevant bioactive constituents in the aqueous extract. The aqueous extraction from licorice inhibited the amplitude and frequency of uterine contraction in a concentration-dependent manner. A morphological examination showed that myometrial smooth muscle cells of oxytocin-stimulated group were oval-shaped and arranged irregularly, while those with a single centrally located nucleus of control and licorice-treated groups were fusiform and arranged orderly. The percentage of phosphorylation of HSP27 at Ser-15 residue increased up to 50.33% at 60 min after oxytocin stimulation. Furthermore, this increase was significantly suppressed by licorice treatment at the concentration of 0.2 and 0.4 mg/mL. Colocalization between HSP27 and α-SMA was observed in the myometrial tissues, especially along the actin bundles in the oxytocin-stimulated group. On the contrary, the colocalization was no longer shown after treatment with licorice. Additionally, employing ChemGPS-NP provided support for a preliminary assignment of liquiritigenin and isoliquiritigenin as protein kinase C (PKC) inhibitors in addition to liquiritigenin, isoliquiritigenin, liquiritin and isoliquiritin as MAPK-activated protein kinase 2 (MK2) inhibitors. These assigned compounds were docked with corresponding crystal structures of respective proteins with negative and low binding energy, which indicated a high affinity and tight binding capacity for the active site of the kinases. These results suggest that licorice exerts its spasmolytic effect through inhibiting the phosphorylation of HSP27 to alter the interaction between HSP27 and actin. Furthermore, our results provide support for the prediction that potential bioactive constituents from aqueous licorice extract inhibit the relevant up-stream kinases that phosphorylate HSP27.
Keywords: ChemGPS-NP prediction; HSP27 phosphorylation; licorice; molecular docking; uterine contraction.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Figure 1
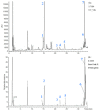
UHPLC-Q Extractive Orbitrap-HRMS Chromatograms of the licorice aqueous extract. The seven peaks correspond to (1) liquiritin apioside or isomer; (2) liquiritin; (3) isoliquiritin apioside; (4) isoliquiritin; (5) liquiritigenin; (6) isoliquiritigenin and (7) glycyrrhetinic acid.
Figure 2
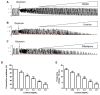
Effects of licorice aqueous extract on oxytocin-stimulated uterine contraction ex vivo: (A) Original tracing example of contractions stimulated by oxytocin (0.01 U/mL) in isolated mouse uterus with DMSO treatment (0.02–0.2%); (B) Original tracing example of relaxant effects of licorice aqueous extract (0.05–0.8 mg/mL) on oxytocin-stimulated contractions; (C) Original tracing example of relaxant effects of nifedipine (positive control, 0.125–1 ng/mL) on oxytocin-stimulated contractions; (D) Concentration-dependent effects of licorice on the contraction amplitude; (E) Concentration-dependent effects of licorice on the contraction frequency. Each column represents the mean ± SD (n = 6). * = p < 0.05, ** = p < 0.01 for the licorice-treated group vs. oxytocin-stimulated group. Statistical significance was analyzed by using one-way ANOVA followed by Tukey’s Honestly Significant Difference test.
Figure 3
Effects of licorice aqueous extract on morphological changes in the uterine inner annular layer. Example histological section of the uterine inner annular layer using HE staining under the light microscope at 200× objective. No discernible pathological changes were observed in the control group and the myometrial cells were slender with round nuclei, which was described as fusiform nucleated cells. The short rod-shaped cells with shorter nuclei were in contractile status, which was described as oval nucleated cells. The licorice aqueous extract downregulated the proportion of oval nucleated cells (contractile cells) in uterine inner annular layer.
Figure 4
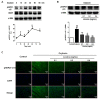
Effects of licorice aqueous extract on phosphorylated HSP27 expression in oxytocin-stimulated uterus. (A) Example of a Western blot analysis of phosphorylated HSP27 (p-HSP27-s15), total HSP27 and α-SMA proteins in uterus at six time points after oxytocin stimulation. Data are presented as the mean ± SD (n = 3) for each time point. ** = p < 0.01 for the oxytocin-stimulated group at 60 min vs. oxytocin-stimulated group at 0 min. Statistical significance was analyzed by using one-way ANOVA followed by Tukey’s Honestly Significant Difference test; (B) Example of a Western blot analysis of phosphorylated HSP27 (p-HSP27-s15) proteins with an internal control of α-SMA in uterus of each treatment group. Data illustrated on the graph bar represent the mean ± SD (n = 3). ## = p < 0.01 for the oxytocin-stimulated model group vs. control group; * = p < 0.05 and ** = p < 0.01 for the licorice-treated group vs. oxytocin-stimulated group. Statistical significance was analyzed by using one-way ANOVA followed by Tukey’s Honestly Significant Difference test; (C) Representative immunofluorescence microscope images of p-HSP27-s15 (green) with DAPI-stained nuclei (blue) in the myometrial tissues were generated using Leica Microsystems (Leica DM2500, Wetzlar, Germany) with a scale bar of 10 μM (n = 3).
Figure 5
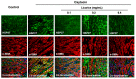
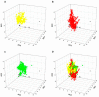
Effects of licorice aqueous extract on the colocalization of HSP27 with α-SMA in oxytocin-stimulated uterus. Representative immunofluorescence microscope images of HSP27 (green), α-SMA (red) and colocalization regions (yellow) with DAPI-stained nuclei (blue) in the myometrial tissues were generated using Leica Microsystems (Leica DM2500, Wetzlar, Germany) with a scale bar of 20 μM (n = 3).
Figure 6
ChemGPS-NP analysis of seven chemical ingredients from the licorice aqueous extract. Score plot of the three dimensions consisting of PC1, PC2 and PC3 from analysis of seven ingredients from licorice aqueous extract: (1) liquiritin apioside; (2) liquiritin; (3) isoliquiritin apioside; (4) isoliquiritin; (5) liquiritigenin; (6) isoliquiritigenin and (7) glycyrrhetinic acid as black triangles with a reference set of known inhibitors targeting p38 (yellow), MK2 (red) and PKC (green) in the ChemGPS-NP model. (A) The seven chemical ingredients (black triangles) were positioned in the resulting map of known p38 inhibitors (yellow); (B) The seven chemical ingredients (black triangles) were positioned in the resulting map of known MK2 inhibitors (red); (C) The seven chemical ingredients (black triangles) were positioned in the resulting map of known PKC inhibitors (green); (D) The seven chemical ingredients (black triangles) were positioned in the resulting map of known p38 (yellow), MK2 (red) and PKC (green) inhibitors.
Figure 7
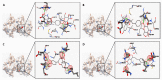
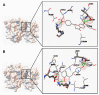
Ligand interaction and comparative binding sites diagrams of the potent ATP competitive inhibitors of MK2: (A) liquiritin; (B) isoliquiritin; (C) liquiritigenin and (D) isoliquiritigenin.
Figure 8
Ligand interaction and comparative binding sites diagrams of (A) liquiritigenin and (B) isoliquiritigenin in the active site of catalytic domain of PKC.
Figure 9
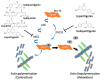
Schematic representation of a model for spasmolytic mechanism of bioactive constituents from aqueous licorice extract on uterine contraction through inhibiting the phosphorylation of heat shock protein 27.
Similar articles
- Relaxative effect of core licorice aqueous extract on mouse isolated uterine horns.
Jia J, Li Y, Lei Z, Hao Y, Wu Y, Zhao Q, Wang H, Ma L, Liu J, Zhao C, Jiang Y, Wang Y, Tan H, Dai X, Zhang W, Sun T, Yu J. Jia J, et al. Pharm Biol. 2013 Jun;51(6):744-8. doi: 10.3109/13880209.2013.764536. Epub 2013 Mar 25. Pharm Biol. 2013. PMID: 23527896 - Isoliquiritigenin, one of the antispasmodic principles of Glycyrrhiza ularensis roots, acts in the lower part of intestine.
Sato Y, He JX, Nagai H, Tani T, Akao T. Sato Y, et al. Biol Pharm Bull. 2007 Jan;30(1):145-9. doi: 10.1248/bpb.30.145. Biol Pharm Bull. 2007. PMID: 17202675 - Analgesic and uterine relaxant effects of isoliquiritigenin, a flavone from Glycyrrhiza glabra.
Shi Y, Wu D, Sun Z, Yang J, Chai H, Tang L, Guo Y. Shi Y, et al. Phytother Res. 2012 Sep;26(9):1410-7. doi: 10.1002/ptr.3715. Epub 2012 Mar 3. Phytother Res. 2012. PMID: 22389128 - History and the immunostimulatory effects of heat-processed licorice root products with or without honey.
Ota M, Makino T. Ota M, et al. J Ethnopharmacol. 2022 Jun 28;292:115108. doi: 10.1016/j.jep.2022.115108. Epub 2022 Feb 18. J Ethnopharmacol. 2022. PMID: 35189279 Review. - Chemical analysis of the Chinese herbal medicine licorice (Gan-Cao): An update review.
Shang Z, Liu C, Qiao X, Ye M. Shang Z, et al. J Ethnopharmacol. 2022 Dec 5;299:115686. doi: 10.1016/j.jep.2022.115686. Epub 2022 Sep 5. J Ethnopharmacol. 2022. PMID: 36067839 Review.
Cited by
- Briaviolides K-N, New Briarane-Type Diterpenoids from Cultured Octocoral Briareum violaceum.
Xu JH, Lai KH, Su YD, Chang YC, Peng BR, Backlund A, Wen ZH, Sung PJ. Xu JH, et al. Mar Drugs. 2018 Feb 27;16(3):75. doi: 10.3390/md16030075. Mar Drugs. 2018. PMID: 29495481 Free PMC article. - A single epidural administration of butorphanol combined with patient-controlled intravenous analgesia relieves postpartum contraction pain after cesarean section: A randomized clinical trial.
Qiu L, Chen XH, Fu J, Hua L, Fan D, Zhang YY, Wang XB. Qiu L, et al. Medicine (Baltimore). 2024 Dec 27;103(52):e40887. doi: 10.1097/MD.0000000000040887. Medicine (Baltimore). 2024. PMID: 39969329 Free PMC article. Clinical Trial. - Xiaoyaosan prevents atherosclerotic vulnerable plaque formation through heat shock protein/glucocorticoid receptor axis-mediated mechanism.
Fu W, Chen M, Ou L, Li T, Chang X, Huang R, Zhang J, Zhang Z. Fu W, et al. Am J Transl Res. 2019 Sep 15;11(9):5531-5545. eCollection 2019. Am J Transl Res. 2019. PMID: 31632527 Free PMC article. - Bioactive Candy: Effects of Licorice on the Cardiovascular System.
Deutch MR, Grimm D, Wehland M, Infanger M, Krüger M. Deutch MR, et al. Foods. 2019 Oct 14;8(10):495. doi: 10.3390/foods8100495. Foods. 2019. PMID: 31615045 Free PMC article. Review. - Traditional Chinese Medicine Targeting Heat Shock Proteins as Therapeutic Strategy for Heart Failure.
Wang Y, Wu J, Wang D, Yang R, Liu Q. Wang Y, et al. Front Pharmacol. 2022 Jan 18;12:814243. doi: 10.3389/fphar.2021.814243. eCollection 2021. Front Pharmacol. 2022. PMID: 35115946 Free PMC article. Review.
References
- Akerlund M., Melin P., Maggi M. Potential use of oxytocin and vasopressin V1a antagonists in the treatment of preterm labour and primary dysmenorrhoea. Adv. Exp. Med. Biol. 1995;395:595–600. - PubMed
- Gusev N.B., Bogatcheva N.V., Marston S.B. Structure and properties of small heat shock proteins (sHsp) and their interaction with cytoskeleton proteins. Biochemistry. 2002;67:511–519. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous