The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing - PubMed (original) (raw)
. 2017 Aug 29;8(4):e00812-17.
doi: 10.1128/mBio.00812-17.
Ann Marie Simmons 2, Mazhgan Rowneki 3, Heta Parmar 3, Yuan Cao 3, Jamie Ryan 2, Padmapriya P Banada 3, Srinidhi Deshpande 3, Shubhada Shenai 3, Alexander Gall 4, Jennifer Glass 2, Barry Krieswirth 5, Samuel G Schumacher 6, Pamela Nabeta 6, Nestani Tukvadze 7, Camilla Rodrigues 8, Alena Skrahina 9, Elisa Tagliani 10, Daniela M Cirillo 10, Amy Davidow 11, Claudia M Denkinger 6, David Persing 2, Robert Kwiatkowski 2, Martin Jones 2, David Alland 1
Affiliations
- PMID: 28851844
- PMCID: PMC5574709
- DOI: 10.1128/mBio.00812-17
The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing
Soumitesh Chakravorty et al. mBio. 2017.
Abstract
The Xpert MTB/RIF assay (Xpert) is a rapid test for tuberculosis (TB) and rifampin resistance (RIF-R) suitable for point-of-care testing. However, it has decreased sensitivity in smear-negative sputum, and false identification of RIF-R occasionally occurs. We developed the Xpert MTB/RIF Ultra assay (Ultra) to improve performance. Ultra and Xpert limits of detection (LOD), dynamic ranges, and RIF-R rpoB mutation detection were tested on Mycobacterium tuberculosis DNA or sputum samples spiked with known numbers of M. tuberculosis H37Rv or Mycobacterium bovis BCG CFU. Frozen and prospectively collected clinical samples from patients suspected of having TB, with and without culture-confirmed TB, were also tested. For M. tuberculosis H37Rv, the LOD was 15.6 CFU/ml of sputum for Ultra versus 112.6 CFU/ml of sputum for Xpert, and for M. bovis BCG, it was 143.4 CFU/ml of sputum for Ultra versus 344 CFU/ml of sputum for Xpert. Ultra resulted in no false-positive RIF-R specimens, while Xpert resulted in two false-positive RIF-R specimens. All RIF-R-associated M. tuberculosis rpoB mutations tested were identified by Ultra. Testing on clinical sputum samples, Ultra versus Xpert, resulted in an overall sensitivity of 87.5% (95% confidence interval [CI], 82.1, 91.7) versus 81.0% (95% CI, 74.9, 86.2) and a sensitivity on sputum smear-negative samples of 78.9% (95% CI, 70.0, 86.1) versus 66.1% (95% CI, 56.4, 74.9). Both tests had a specificity of 98.7% (95% CI, 93.0, 100), and both had comparable accuracies for detection of RIF-R in these samples. Ultra should significantly improve TB detection, especially in patients with paucibacillary disease, and may provide more-reliable RIF-R detection.IMPORTANCE The Xpert MTB/RIF assay (Xpert), the first point-of-care assay for tuberculosis (TB), was endorsed by the World Health Organization in December 2010. Since then, 23 million Xpert tests have been procured in 130 countries. Although Xpert showed high overall sensitivity and specificity with pulmonary samples, its sensitivity has been lower with smear-negative pulmonary samples and extrapulmonary samples. In addition, the prediction of rifampin resistance (RIF-R) in paucibacillary samples and for a few rpoB mutations has resulted in both false-positive and false-negative results. The present study is the first demonstration of the design features and operational characteristics of an improved Xpert Ultra assay. This study also shows that the Ultra format overcomes many of the known shortcomings of Xpert. The new assay should significantly improve TB detection, especially in patients with paucibacillary disease, and provide more-reliable detection of RIF-R.
Keywords: Xpert MTB/RIF Ultra; diagnosis; sensitive; tuberculosis.
Copyright © 2017 Chakravorty et al.
Figures
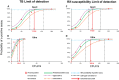
FIG 1
Limit of detection for M. tuberculosis H37Rv. The limit of detection of tuberculosis detection is shown for Xpert (A) versus Ultra (B). The limit of detection for generating a rifampin susceptibility rather than an indeterminate result is shown for Xpert (C) versus Ultra (D).
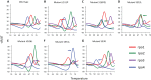
FIG 2
Detection of M. tuberculosis rpoB gene mutations associated with rifampin resistance. Derivative-transformed curves of four _rpoB_-specific sloppy molecular beacons (rpo1, rpo2, rpo3, and rpo4) first hybridized and then melted off of their M. tuberculosis rpoB gene amplicon target are shown. Each colored peak indicates the melting temperature of the probe corresponding to the colored line. The peaks for wild-type M. tuberculosis (A) identify a test sample as rifampin susceptible. The shift in one or more of the peaks away from the wild type’s melting temperature (B to G), identify a sample as an rpoB mutant and rifampin resistant. The shift in the melt peak is indicated by arrows.
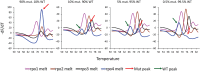
FIG 3
Detection of hetero-resistance. Samples containing equal quantities of total M. tuberculosis DNA were created using different proportions of wild-type and rifampin-resistant rpoB S531L mutant DNA. Derivative-transformed curves of four _rpoB_-specific sloppy molecular beacons (rpo1, rpo2, rpo3, and rpo4) first hybridized and then melted off of their M. tuberculosis rpoB gene amplicon target are shown. The presence of rifampin-resistant mutant DNA, as indicated by the peak marked with a red arrow, was consistently detected in samples containing as little as 10% mutant DNA and in a subset of samples containing as little as 5% mutant DNA. WT, wild type; mut., mutant.
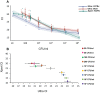
FIG 4
Comparative dynamic range of Ultra and Xpert. Log dilutions of M. tuberculosis H37Rv spiked into sputum samples (minimum of 20 samples per dilution) were tested by Ultra and Xpert. The same dilutions of BCG were also tested by Ultra. (A) The C T values for each CFU dilution are shown. Colored circles show the C T of each positive result. Assay results that were negative are not shown, as these did not result in any C T value. Colored shading shows ± 1 standard deviation. (B) The experimental results for the H37Rv dynamic-range study comparing the mean C T values of each log dilution produced by Ultra versus Xpert are also shown. Error bars show ± 1 standard deviation.
Similar articles
- Evaluation of Xpert MTB/RIF Ultra performance for pulmonary tuberculosis diagnosis on smear-negative respiratory samples in a French centre.
Hodille E, Maisson A, Charlet L, Bauduin C, Genestet C, Fredenucci I, Rasigade JP, Lina G, Dumitrescu O. Hodille E, et al. Eur J Clin Microbiol Infect Dis. 2019 Mar;38(3):601-605. doi: 10.1007/s10096-018-03463-1. Epub 2019 Jan 24. Eur J Clin Microbiol Infect Dis. 2019. PMID: 30680567 - Comparison of targeted next-generation sequencing and the Xpert MTB/RIF assay for detection of Mycobacterium tuberculosis in clinical isolates and sputum specimens.
Zhang H, Dai X, Hu P, Tian L, Li C, Ding B, Yang X, He X. Zhang H, et al. Microbiol Spectr. 2024 May 2;12(5):e0409823. doi: 10.1128/spectrum.04098-23. Epub 2024 Apr 11. Microbiol Spectr. 2024. PMID: 38602399 Free PMC article. - [Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary tuberculosis in an intermediate-prevalence setting].
Ozkutuk N, Surucüoglu S. Ozkutuk N, et al. Mikrobiyol Bul. 2014 Apr;48(2):223-32. doi: 10.5578/mb.7456. Mikrobiyol Bul. 2014. PMID: 24819260 Turkish. - Xpert MTB/RIF and Xpert Ultra assays for screening for pulmonary tuberculosis and rifampicin resistance in adults, irrespective of signs or symptoms.
Shapiro AE, Ross JM, Yao M, Schiller I, Kohli M, Dendukuri N, Steingart KR, Horne DJ. Shapiro AE, et al. Cochrane Database Syst Rev. 2021 Mar 23;3(3):CD013694. doi: 10.1002/14651858.CD013694.pub2. Cochrane Database Syst Rev. 2021. PMID: 33755189 Free PMC article. - Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children.
Kay AW, González Fernández L, Takwoingi Y, Eisenhut M, Detjen AK, Steingart KR, Mandalakas AM. Kay AW, et al. Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD013359. doi: 10.1002/14651858.CD013359.pub2. Cochrane Database Syst Rev. 2020. PMID: 32853411 Free PMC article. Updated.
Cited by
- Evolution of Mycobacterium tuberculosis drug resistance in the genomic era.
Nimmo C, Millard J, Faulkner V, Monteserin J, Pugh H, Johnson EO. Nimmo C, et al. Front Cell Infect Microbiol. 2022 Oct 7;12:954074. doi: 10.3389/fcimb.2022.954074. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36275027 Free PMC article. Review. - The clinical and molecular diagnosis of childhood and adolescent pulmonary tuberculosis in referral centers.
Aurilio RB, Luiz RR, Land MGP, Cardoso CAA, Kritski AL, Sant'Anna CC. Aurilio RB, et al. Rev Soc Bras Med Trop. 2020 Sep 25;53:e20200205. doi: 10.1590/0037-8682-0205-2020. eCollection 2020. Rev Soc Bras Med Trop. 2020. PMID: 32997050 Free PMC article. - Diagnostic value of tNGS vs Xpert MTB/RIF in childhood TB.
Zheng H, Yang H, Wang Y, Li F, Xiao J, Guo Y, Chen H, Wang X, Li H, Shen C. Zheng H, et al. Heliyon. 2023 Dec 3;10(1):e23217. doi: 10.1016/j.heliyon.2023.e23217. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38148816 Free PMC article. - Usefulness of Xpert MTB/RIF Ultra to Rapidly Diagnose Sputum Smear-Negative Pulmonary Tuberculosis Using Bronchial Washing Fluid.
Chien JY, Lin CK, Yu CJ, Hsueh PR. Chien JY, et al. Front Microbiol. 2020 Sep 22;11:588963. doi: 10.3389/fmicb.2020.588963. eCollection 2020. Front Microbiol. 2020. PMID: 33072058 Free PMC article. - Rapid Phenotypic and Genotypic Antimicrobial Susceptibility Testing Approaches for Use in the Clinical Laboratory.
Hattab S, Ma AH, Tariq Z, Vega Prado I, Drobish I, Lee R, Yee R. Hattab S, et al. Antibiotics (Basel). 2024 Aug 22;13(8):786. doi: 10.3390/antibiotics13080786. Antibiotics (Basel). 2024. PMID: 39200086 Free PMC article. Review.
References
- Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, Safi H, Blakemore R, Lan NT, Jones-López EC, Levi M, Burday M, Ayakaka I, Mugerwa RD, McMillan B, Winn-Deen E, Christel L, Dailey P, Perkins MD, Persing DH, Alland D. 2010. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol 48:229–237. doi: 10.1128/JCM.01463-09. - DOI - PMC - PubMed
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, Allen J, Tahirli R, Blakemore R, Rustomjee R, Milovic A, Jones M, O’Brien SM, Persing DH, Ruesch-Gerdes S, Gotuzzo E, Rodrigues C, Alland D, Perkins MD. 2010. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1015. doi: 10.1056/NEJMoa0907847. - DOI - PMC - PubMed
- Lawn SD, Brooks SV, Kranzer K, Nicol MP, Whitelaw A, Vogt M, Bekker LG, Wood R. 2011. Screening for HIV-associated tuberculosis and rifampicin resistance before antiretroviral therapy using the Xpert MTB/RIF assay: a prospective study. PLoS Med 8:e1001067. doi: 10.1371/journal.pmed.1001067. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical