Immunization with alkyl hydroperoxide reductase subunit C reduces Fusobacterium nucleatum load in the intestinal tract - PubMed (original) (raw)
Immunization with alkyl hydroperoxide reductase subunit C reduces Fusobacterium nucleatum load in the intestinal tract
Song-He Guo et al. Sci Rep. 2017.
Abstract
Fusobacterium nucleatum (Fn) is an important tumour-associated bacterium in colorectal cancer (CRC). The antioxidant protein alkyl hydroperoxide reductase subunit C (AhpC) can induce strong antibacterial immune response during various pathogen infections. Our study aimed to evaluate the efficacy of Fn-AhpC as a candidate vaccine. In this work, by western blot analysis, we showed that Fn-AhpC recombinant protein could be recognized specifically by antibodies present in the sera of CRC patients; using the mouse Fn-infection model, we observed that systemic prophylactic immunization with AhpC/alum conferred significant protection against infection in 77.3% of mice. In addition, we measured the anti-AhpC antibody level in the sera of CRC patients and found that there was no obvious increase of anti-AhpC antibodies in the early-stage CRC group. Furthermore, we treated Fn with the sera from both immunized mice and CRC patients and found that sera with high anti-AhpC antibodies titre could inhibit Fn growth. In conclusion, our findings support the use of AhpC as a potential vaccine candidate against inhabitation or infection of Fn in the intestinal tract, which could provide a practical strategy for the prevention of CRC associated with Fn infection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
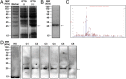
Preparation of recombinant _F. nucleatum_-AhpC and identification of its immunogenic role in CRC patients. (A) Expression of recombinant Fn_-_AhpC after IPTG induction. The PCR amplified AhpC-DNA was inserted into pET28a vector before transformed into E. Coli BL21 strain. The expression of recombinant AhpC in the absence (−) or presence (+) of 0.5 mM IPTG was detected using 12% SDS-PAGE and Coomassie brilliant blue staining. (B) Purification of recombinant AhpC. The recombinant AhpC with a His tag was purified using a Ni-NTA column. (C) Identification of recombinant AhpC. The recombinant AhpC proteins were extracted from the gels stained with Coomassie brilliant blue R250, and subsequently digested with trypsin. The resulting peptides were further analysed using a MALDI-TOF/TOF analyser. (D) Antigens reactive with anti_-_AhpC-IgA were determined using western blotting. Recombinant AhpC were incubated with a reference dilution of pooled serum from 6 healthy subjects or separated serum from 6 Fn-positive CRC individuals as primary antibody. Notably, Fig. 1A,B and D were cropped from a single image on the dashed or solid lines to be better presented in the article’s context. The complete figures for Fig. 1A,B and D are provided in Supplementary Fig. 1A, Supplementary Fig. 1B and Supplementary Fig. 2, respectively.
Figure 2
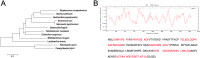
Phylogenetic tree analysis and antigenic determinants prediction for AhpC of F. nucleatum. (A) Phylogenetic tree of AhpC amino acid sequences. The amino acid sequences of AhpC present in protein database of GenBank were analysed using MEGA version 5.1 (
). The numbers at each branch represent the bootstrap values obtained with 1000 replicates. (B) The improved self-optimized software (DNAStar Protean system) was used to predict the antigenic plot for Fn-AhpC protein. Average antigenic propensity for this protein is 1.0330.
Figure 3
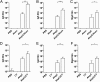
Quantitative antibody response to _F. nucleatum_-AhpC immunization. Mice were immunized by intraperitoneal injection (systemic immunization) (A,B,C) or by intragastric administration (mucosal immunization) (D,E,F) with PBS, adjuvant, Fn-AhpC or Fn-AhpC combined with alum or cholera toxin (CT). One week after final vaccination, anti-AhpC IgG and IgA titres in sera/intestinal mucus were determined using ELISA. *P < 0.05, **P < 0.01.
Figure 4
Induction of significant protection against F. nucleatum by immunization with _F. nucleatum_-AhpC vaccine. Mice were immunized by intraperitoneal injection (A) or intragastric administration (B) with PBS, AhpC or AhpC combined with alum or cholera toxin (CT). One week after the final vaccination, the mice were challenged with F. nucleatum and colonization quantified using qPCR assay. *P < 0.05, **P < 0.01.
Figure 5
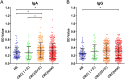
Comparison of serum levels of anti_-F. nucleatum_-AhpC from CRC patients and healthy subjects. Healthy subjects (HS, n = 92), stage I-II of CRC (n = 55), stage III-IV of CRC (n = 203), the total of CRC patients (n = 258) were individually assayed. Symbols indicate individual OD value; horizontal lines indicate the mean values ± SD. Differences between the four groups were analysed using the Kruskal-Wallis test. *P < 0.05. (A) anti-Fn-AhpC-IgA. (B) anti-Fn-AhpC-IgG.
Figure 6
Serum with high titre antibodies to AhpC inhibited Fn growth in vitro. Survival of F. nucleatum strains was measured by bactericidal assays in serum of AhpC immunized and unimmunized mice (A) or CRC patients and healthy subjects (B). Inhibition of F. nucleatum strains was measured using antibacterial assays in the serum of AhpC immunized and unimmunized mice (C) or CRC patients and healthy subjects (D). Flow cytometric analysis of the stained Fn (E). Gates indicate the position and concentration of intact cells on the plots. Q1: dead cells, Q2: live cells; Q3: injured cells and debris. *P < 0.05, **P < 0.01, ns: no significant.
Similar articles
- Fn-Dps, a novel virulence factor of Fusobacterium nucleatum, disrupts erythrocytes and promotes metastasis in colorectal cancer.
Wu Y, Guo S, Chen F, Li Y, Huang Y, Liu W, Zhang G. Wu Y, et al. PLoS Pathog. 2023 Jan 24;19(1):e1011096. doi: 10.1371/journal.ppat.1011096. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36693067 Free PMC article. - Alkyl hydroperoxide reductase: a candidate Helicobacter pylori vaccine.
O'Riordan AA, Morales VA, Mulligan L, Faheem N, Windle HJ, Kelleher DP. O'Riordan AA, et al. Vaccine. 2012 Jun 6;30(26):3876-84. doi: 10.1016/j.vaccine.2012.04.002. Epub 2012 Apr 15. Vaccine. 2012. PMID: 22512976 - Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF-_κ_B/S100A9 Cascade.
Hu L, Liu Y, Kong X, Wu R, Peng Q, Zhang Y, Zhou L, Duan L. Hu L, et al. Front Immunol. 2021 May 21;12:658681. doi: 10.3389/fimmu.2021.658681. eCollection 2021. Front Immunol. 2021. PMID: 34093546 Free PMC article. - _Fusobacterium nucleatum_-positive colorectal cancer.
Yang Z, Ji G. Yang Z, et al. Oncol Lett. 2019 Aug;18(2):975-982. doi: 10.3892/ol.2019.10433. Epub 2019 Jun 4. Oncol Lett. 2019. PMID: 31423156 Free PMC article. Review.
Cited by
- Fn-Dps, a novel virulence factor of Fusobacterium nucleatum, disrupts erythrocytes and promotes metastasis in colorectal cancer.
Wu Y, Guo S, Chen F, Li Y, Huang Y, Liu W, Zhang G. Wu Y, et al. PLoS Pathog. 2023 Jan 24;19(1):e1011096. doi: 10.1371/journal.ppat.1011096. eCollection 2023 Jan. PLoS Pathog. 2023. PMID: 36693067 Free PMC article. - Tubeimuside I improves the efficacy of a therapeutic Fusobacterium nucleatum dendritic cell-based vaccine against colorectal cancer.
Tong Y, Lu G, Wang Z, Hao S, Zhang G, Sun H. Tong Y, et al. Front Immunol. 2023 May 3;14:1154818. doi: 10.3389/fimmu.2023.1154818. eCollection 2023. Front Immunol. 2023. PMID: 37207216 Free PMC article. - Development of Melioidosis Subunit Vaccines Using an Enzymatically Inactive Burkholderia pseudomallei AhpC.
Schmidt LK, Orne CE, Shaffer TL, Wilson SM, Khakhum N, Torres AG, Brett PJ, Burtnick MN. Schmidt LK, et al. Infect Immun. 2022 Aug 18;90(8):e0022222. doi: 10.1128/iai.00222-22. Epub 2022 Jul 11. Infect Immun. 2022. PMID: 35862715 Free PMC article. - Targeting Programmed Fusobacterium nucleatum Fap2 for Colorectal Cancer Therapy.
Ganesan K, Guo S, Fayyaz S, Zhang G, Xu B. Ganesan K, et al. Cancers (Basel). 2019 Oct 18;11(10):1592. doi: 10.3390/cancers11101592. Cancers (Basel). 2019. PMID: 31635333 Free PMC article. Review. - Molecular Mechanisms of AhpC in Resistance to Oxidative Stress in Burkholderia thailandensis.
Zhang B, Gu H, Yang Y, Bai H, Zhao C, Si M, Su T, Shen X. Zhang B, et al. Front Microbiol. 2019 Jul 2;10:1483. doi: 10.3389/fmicb.2019.01483. eCollection 2019. Front Microbiol. 2019. PMID: 31338075 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous