Local Nucleation of Microtubule Bundles through Tubulin Concentration into a Condensed Tau Phase - PubMed (original) (raw)
Local Nucleation of Microtubule Bundles through Tubulin Concentration into a Condensed Tau Phase
Amayra Hernández-Vega et al. Cell Rep. 2017.
Abstract
Non-centrosomal microtubule bundles play important roles in cellular organization and function. Although many diverse proteins are known that can bundle microtubules, biochemical mechanisms by which cells could locally control the nucleation and formation of microtubule bundles are understudied. Here, we demonstrate that the concentration of tubulin into a condensed, liquid-like compartment composed of the unstructured neuronal protein tau is sufficient to nucleate microtubule bundles. We show that, under conditions of macro-molecular crowding, tau forms liquid-like drops. Tubulin partitions into these drops, efficiently increasing tubulin concentration and driving the nucleation of microtubules. These growing microtubules form bundles, which deform the drops while remaining enclosed by diffusible tau molecules exhibiting a liquid-like behavior. Our data suggest that condensed compartments of microtubule bundling proteins could promote the local formation of microtubule bundles in neurons by acting as non-centrosomal microtubule nucleation centers and that liquid-like tau encapsulation could provide both stability and plasticity to long axonal microtubule bundles.
Keywords: cytoskeleton; drops; intrinsically disordered proteins; liquid-like; microtubule bundles; multivalency; nucleation; phase separation; tau; tubulin.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1. Tau Phase Separates into Liquid-like Drops
(A) Prediction of the degree of disorder along tau protein (htau441 isoform). PONDR-FIT (P-FIT) and VL3 algorithms are shown in blue and orange, respectively. A given region is considered disordered when the disorder probability is above 0.5. Low complexity domain (LCD)1 and LCD2 (pale blue rectangles) highlight regions of the protein with potential low complexity. Tau protein domains are shown below: NT-PD, N-terminal projection domain; PRD, proline-rich domain; TB1–TB4, tubulin binding repeats 1 to 4; CT-PD, C-terminal projection domain. (B) Tau forms drops in vitro in the presence of 10% of crowding agent (dextran, T500). Bright-field and fluorescence microscopy images of tau-EGFP drops. See also Figure S2. Tau drops were formed with 25 µM tau-EGFP, 25 mM HEPES, 150 mM KCl, 1 mM DTT, and 10% dextran (pH 7.4) for all experiments in this figure. Recombinant tau was purified from insect cells. See SDS gel of purified proteins in Figure S1. (C) Fusion of tau droplets using dual-trap optical tweezers. Top panel: time course of the fusion event (bright-field image) aligned to the laser signal (lower plot) recorded during fusion relaxation. See also Movie S1. The combined signal of the two traps is shown in the graph. Data were fit with two exponentials (magenta line). The τ constant (gray rectangle) for 26 fusion events of the fast, initial relaxation was plotted against the characteristic length of the droplet using the geometric radius (left inlet graph) to extract the ratio of dynamic viscosity to surface tension (slope). Data were fit with a robust linear fit (magenta line in inlet). Note the representation of 2 outliners by arrows, with their position indicated. (D) Internal rearrangement of tau drops. Time course of fluorescence recovery after photo-bleaching (FRAP) after internal photo-bleaching of tau drops. LUT, Fiji 16 colors. See also Movie S2. Bottom panel: plot of the recovery in the photo-bleached area. Values shown are the mean ± SD, n = 17. Time 0 indicates the time for the photo-bleaching. Values were normalized to the first time point before photo-bleaching. (E) Tau drop deformation by shear flow. Snapshots of Movie S3. Shear flow was applied from the top left to the bottom right of the image.
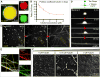
Figure 2. Tau Drops Concentrate Tubulin and Polymerize Tau-Encapsulated Microtubule Bundles
(A) Concentration of tubulin into tau drops. Merged and single-channel fluorescence microscopy images of tau drops (green) with incorporated tubulin dimers (red) 5min after mixing. An overall concentration of 5 µM rhodamine-labeled tubulin was added to tau drops. Tau drops were formed with 25 µM tau-EGFP in 13 BRB80 (pH 6.9) with 1 mM DTT and 10% dextran in all experiments with tubulin, if not otherwise mentioned. (B) Tubulin partition coefficient quantified by the ratio of the mean intensity inside the drops to the mean intensity in the surrounding bulk media at different concentrations of overall tubulin (no GTP added). Values shown are the mean ± SD; n = 16 image stacks, 50 images per stack, mean of all drops in the stack. The concentration of tubulin in tau drops was above 10-fold in all concentrations of tubulin tested. See also Figure S3. (C) Tau drop deformation by internal microtubule bundle polymerization. 5 µM rhodamine-tubulin were added to tau drops, together with 1 mM GTP. Immediately after the addition of tubulin, the drops deformed. See also Movie S4. All experiments in this paper were performed at room temperature (RT). (D) Detail from the previous panel of a single drop deformation upon addition of tubulin and GTP. Drops deformed bidirectionally due to the polymerization of internal nucleated microtubule bundles. Drops redistributed their volume along the growing microtubule bundles. See also Movie S5. (E) Tau and tubulin co-localization in tau-encapsulated bundles formed from the deforming drops. Single-channel and merged maximum projection images are shown. The concentrations and conditions used are the same as in previous panels. (F) Nucleation of tau-encapsulated bundles at low overall tubulin concentrations, 15 min after addition of tubulin. 0.75 µM of overall tubulin is sufficient to deform some drops by the internally nucleated microtubule bundles. Stitching of 16 maximum projection images is shown in each panel. Rhodamine-labeled tubulin was added at the indicated concentration to preformed tau drops.
Figure 3. Tau in Tau-Encapsulated Microtubule Bundles Displays Liquid-like Properties
(A) Internal rearrangement of tubulin and tau in tau-encapsulated microtubule bundles. Left panel: time course of the recovery of the fluorescent signal after photo-bleaching for both tubulin (left row) and tau (right row) in bundles formed from tau drops. The recovery of 3 photo-bleached rectangular regions (dashed lines) is shown for each case. LUT, Fiji 16 colors. See also Movie S7. Right panel: quantification of the recovery for both tau (green) and tubulin (red) in bundles. Values shown are the mean ± SD; n = 14 (tau) and 19 (tubulin). Bundles were formed from tau drops with the protein concentrations and buffer conditions mentioned in previous figure. (B) Tau-encapsulated microtubule bundle fusion. Time-course snapshots of the fusion of three bundles. See also Movie S8. Maximum projection fluorescence microscopy images of tau-EGFP are shown. Bundles were formed as mentioned earlier. (C) Fusion of tau-mCherry drops to preformed tau-EGFP bundles. Bundles were formed from tau-EGFP drops as before but adding 5 µM of unlabeled tubulin, together with 1 mM GTP. Tau-mCherry drops were formed with 25 µM of tau-mCherry in same buffer conditions and added 1:2 to the bundles. (D) Guided growth of microtubules polymerizing in drops along pre-existing microtubules. See also Movie S9. Tau drops were formed as in previous panels. 5 µM of Cy5-tubulin, together with 1 mM of GTP, were added to tau drops. Cy5-tubulin and GTP-loaded tau drops were flushed into a flow chamber containing immobilized and stable rhodamine-labeled microtubules. Microtubules growing in drops (cyan, Cy5-tubulin) grow in the direction of the adjacent pre-existing microtubule (magenta). Microtubules were double-stabilized using Taxol and GMP-CPP.
Figure 4. Tau Encapsulation Maintains Microtubule Bundles
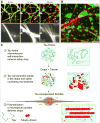
(A) Heparin addition to tau-encapsulated bundles. Upper panels: time course of tau detachment from microtubule bundles, its reshaping back into drops, and the simultaneous debundling of microtubules upon addition of heparin. Tau-EGFP is shown in green, and rhodamine-tubulin is shown in red. See also Movie S10. Lower panel: detail of the microtubule debundling. 200 µg/mL of heparin were added to bundles formed with 5 µM rhodamine-tubulin and 1 mM GTP (protein concentration and buffer conditions as in previous figures). Maximum projection images are shown. (B) Approximately 1 hr after heparin addition, tau is reshaped into both free and microtubule-attached drops. Conditions used are as in the previous panel. (C) Proposed model for tau drop nucleation of microtubule bundles. (1) Tau’s intrinsically disordered properties enable its phase separation in a crowded environment. (2) Tubulin dimers get concentrated inside tau drops, allowing microtubule nucleation inside tau drops. (3) Microtubule bundles grow within drops, deform it into a rod-like shape, and remain surrounded by liquid-like diffusible tau. Tau’s simultaneous binding to multiple tubulin dimers by its four putative tubulin binding repeats, together with its intrinsically disordered arms, may be important for tubulin concentration and microtubule bundle nucleation and stabilization in drops.
Similar articles
- Orientation, assembly, and stability of microtubule bundles induced by a fragment of tau protein.
Brandt R, Lee G. Brandt R, et al. Cell Motil Cytoskeleton. 1994;28(2):143-54. doi: 10.1002/cm.970280206. Cell Motil Cytoskeleton. 1994. PMID: 8087873 - Paclitaxel suppresses Tau-mediated microtubule bundling in a concentration-dependent manner.
Choi MC, Chung PJ, Song C, Miller HP, Kiris E, Li Y, Wilson L, Feinstein SC, Safinya CR. Choi MC, et al. Biochim Biophys Acta Gen Subj. 2017 Jan;1861(1 Pt A):3456-3463. doi: 10.1016/j.bbagen.2016.09.011. Epub 2016 Sep 12. Biochim Biophys Acta Gen Subj. 2017. PMID: 27632200 - Identification of a new microtubule-interacting protein Mip-90.
González M, Cambiazo V, Maccioni RB. González M, et al. Eur J Cell Biol. 1995 Jun;67(2):158-69. Eur J Cell Biol. 1995. PMID: 7664757 - Regulation of microtubule nucleation mediated by γ-tubulin complexes.
Sulimenko V, Hájková Z, Klebanovych A, Dráber P. Sulimenko V, et al. Protoplasma. 2017 May;254(3):1187-1199. doi: 10.1007/s00709-016-1070-z. Epub 2017 Jan 10. Protoplasma. 2017. PMID: 28074286 Review. - Liquid-Liquid Phase Separation of Tau Protein in Neurobiology and Pathology.
Wegmann S. Wegmann S. Adv Exp Med Biol. 2019;1184:341-357. doi: 10.1007/978-981-32-9358-8_25. Adv Exp Med Biol. 2019. PMID: 32096048 Review.
Cited by
- Residue-specific identification of phase separation hot spots of Alzheimer's-related protein tau.
Ambadipudi S, Reddy JG, Biernat J, Mandelkow E, Zweckstetter M. Ambadipudi S, et al. Chem Sci. 2019 May 22;10(26):6503-6507. doi: 10.1039/c9sc00531e. eCollection 2019 Jul 14. Chem Sci. 2019. PMID: 31341602 Free PMC article. - Mechanisms of microtubule organization in differentiated animal cells.
Akhmanova A, Kapitein LC. Akhmanova A, et al. Nat Rev Mol Cell Biol. 2022 Aug;23(8):541-558. doi: 10.1038/s41580-022-00473-y. Epub 2022 Apr 5. Nat Rev Mol Cell Biol. 2022. PMID: 35383336 Review. - A hydrodynamic instability drives protein droplet formation on microtubules to nucleate branches.
Setru SU, Gouveia B, Alfaro-Aco R, Shaevitz JW, Stone HA, Petry S. Setru SU, et al. Nat Phys. 2021 Apr;17(4):493-498. doi: 10.1038/s41567-020-01141-8. Epub 2021 Jan 28. Nat Phys. 2021. PMID: 35211183 Free PMC article. No abstract available. - Phase Separation and Mechanical Forces in Regulating Asymmetric Cell Division of Neural Stem Cells.
Zhang Y, Wei H, Wen W. Zhang Y, et al. Int J Mol Sci. 2021 Sep 24;22(19):10267. doi: 10.3390/ijms221910267. Int J Mol Sci. 2021. PMID: 34638607 Free PMC article. Review. - The variable domain from dynamin-related protein 1 promotes liquid-liquid phase separation that enhances its interaction with cardiolipin-containing membranes.
Posey AE, Ross KA, Bagheri M, Lanum EN, Khan MA, Jennings CE, Harwig MC, Kennedy NW, Hilser VJ, Harden JL, Hill RB. Posey AE, et al. Protein Sci. 2023 Nov;32(11):e4787. doi: 10.1002/pro.4787. Protein Sci. 2023. PMID: 37743569 Free PMC article.
References
- Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res. Bull. 2016;126:238–292. - PubMed
- Baas PW, Karabay A, Qiang L. Microtubules cut and run. Trends Cell Biol. 2005;15:518–524. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources