Brain cortical characteristics of lifetime cognitive ageing - PubMed (original) (raw)
. 2018 Jan;223(1):509-518.
doi: 10.1007/s00429-017-1505-0. Epub 2017 Sep 6.
Simon R Cox 1 2 3, Mark E Bastin 4 5 6, David Alexander Dickie 4 5 6, Dave C Liewald 4 7, Susana Muñoz Maniega 4 5 6, Paul Redmond 7, Natalie A Royle 4 5 6, Alison Pattie 7, Maria Valdés Hernández 4 5 6, Janie Corley 7, Benjamin S Aribisala 4 6 8, Andrew M McIntosh 9, Joanna M Wardlaw 4 5 6, Ian J Deary 4 7
Affiliations
- PMID: 28879544
- PMCID: PMC5772145
- DOI: 10.1007/s00429-017-1505-0
Brain cortical characteristics of lifetime cognitive ageing
Simon R Cox et al. Brain Struct Funct. 2018 Jan.
Abstract
Regional cortical brain volume is the product of surface area and thickness. These measures exhibit partially distinct trajectories of change across the brain's cortex in older age, but it is unclear which cortical characteristics at which loci are sensitive to cognitive ageing differences. We examine associations between change in intelligence from age 11 to 73 years and regional cortical volume, surface area, and thickness measured at age 73 years in 568 community-dwelling older adults, all born in 1936. A relative positive change in intelligence from 11 to 73 was associated with larger volume and surface area in selective frontal, temporal, parietal, and occipital regions (r < 0.180, FDR-corrected q < 0.05). There were no significant associations between cognitive ageing and a thinner cortex for any region. Interestingly, thickness and surface area were phenotypically independent across bilateral lateral temporal loci, whose surface area was significantly related to change in intelligence. These findings suggest that associations between regional cortical volume and cognitive ageing differences are predominantly driven by surface area rather than thickness among healthy older adults. Regional brain surface area has been relatively underexplored, and is a potentially informative biomarker for identifying determinants of cognitive ageing differences.
Keywords: Ageing; Cortex; Intelligence; MRI; Surface area; Thickness.
Conflict of interest statement
The authors declare no competing financial or other conflicts of interests.
Figures
Fig. 1
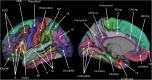
Schematic of parcellated cortical regions according to Desikan et al. (2006). FP frontal pole, DLPF dorsolateral prefrontal, IF inferior frontal, lOrbital lateral orbitofrontal, mOrbital medial orbitofrontal, CMF caudal middle frontal, RACing rostral anterior cingulate, CACing caudal anterior cingulate, TP temporal pole, ST superior temporal, MT middle temporal, IT inferior temporal, ParaHip parahippocampal, SP superior parietal, IP inferior parietal, PCing posterior cingulate, lOccipital lateral occipital, mOccipital medial occipital. See Supplementary Material for further information
Fig. 2
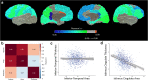
a Regional associations between raw cortical surface area and thickness; b correlation matrix of associations between global volume, surface area, and thickness; c absence of association between cortical surface area and thickness in inferior temporal gyrus; d strongest negative association between cortical surface area and thickness is exhibited in the isthmus cingulate gyrus. For panels c and d, light blue points and solid regression lines (with shaded 95% confidence intervals) denote right sided measures, and dark blue points and broken regression lines indicate left sided measures
Fig. 3
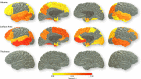
FDR-corrected associations between lifetime cognitive change between 11 and 73 (corrected for age at testing, sex) and brain cortical volume (top row), surface area (middle), and thickness (bottom). Magnitude (Pearson’s r) of association is reported for each regional cortical measure (corrected for age at scan, sex, and intracranial volume)
Similar articles
- Different regional patterns of cortical thinning in Alzheimer's disease and frontotemporal dementia.
Du AT, Schuff N, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin K, Miller BL, Weiner MW. Du AT, et al. Brain. 2007 Apr;130(Pt 4):1159-66. doi: 10.1093/brain/awm016. Epub 2007 Mar 12. Brain. 2007. PMID: 17353226 Free PMC article. - Age-related decline in the brain: a longitudinal study on inter-individual variability of cortical thickness, area, volume, and cognition.
Sele S, Liem F, Mérillat S, Jäncke L. Sele S, et al. Neuroimage. 2021 Oct 15;240:118370. doi: 10.1016/j.neuroimage.2021.118370. Epub 2021 Jul 8. Neuroimage. 2021. PMID: 34245866 - Changes in thickness and surface area of the human cortex and their relationship with intelligence.
Schnack HG, van Haren NE, Brouwer RM, Evans A, Durston S, Boomsma DI, Kahn RS, Hulshoff Pol HE. Schnack HG, et al. Cereb Cortex. 2015 Jun;25(6):1608-17. doi: 10.1093/cercor/bht357. Epub 2014 Jan 9. Cereb Cortex. 2015. PMID: 24408955 - Sex, age, and cognitive correlates of asymmetries in thickness of the cortical mantle across the life span.
Plessen KJ, Hugdahl K, Bansal R, Hao X, Peterson BS. Plessen KJ, et al. J Neurosci. 2014 Apr 30;34(18):6294-302. doi: 10.1523/JNEUROSCI.3692-13.2014. J Neurosci. 2014. PMID: 24790200 Free PMC article. - Fluid intelligence is associated with cortical volume and white matter tract integrity within multiple-demand system across adult lifespan.
Chen PY, Chen CL, Hsu YC; Cam-CAN; Tseng WI. Chen PY, et al. Neuroimage. 2020 May 15;212:116576. doi: 10.1016/j.neuroimage.2020.116576. Epub 2020 Feb 24. Neuroimage. 2020. PMID: 32105883
Cited by
- Neurology-related protein biomarkers are associated with cognitive ability and brain volume in older age.
Harris SE, Cox SR, Bell S, Marioni RE, Prins BP, Pattie A, Corley J, Muñoz Maniega S, Valdés Hernández M, Morris Z, John S, Bronson PG, Tucker-Drob EM, Starr JM, Bastin ME, Wardlaw JM, Butterworth AS, Deary IJ. Harris SE, et al. Nat Commun. 2020 Feb 10;11(1):800. doi: 10.1038/s41467-019-14161-7. Nat Commun. 2020. PMID: 32041957 Free PMC article. - Neuroanatomical norms in the UK Biobank: The impact of allometric scaling, sex, and age.
Williams CM, Peyre H, Toro R, Ramus F. Williams CM, et al. Hum Brain Mapp. 2021 Oct 1;42(14):4623-4642. doi: 10.1002/hbm.25572. Epub 2021 Jul 16. Hum Brain Mapp. 2021. PMID: 34268815 Free PMC article. - Causal effects of cingulate morphology on executive functions in healthy young adults.
Razzaq FA, Bringas Vega ML, Ontiveiro-Ortega M, Riaz U, Valdes-Sosa PA. Razzaq FA, et al. Hum Brain Mapp. 2022 Oct 1;43(14):4370-4382. doi: 10.1002/hbm.25960. Epub 2022 Jun 6. Hum Brain Mapp. 2022. PMID: 35665983 Free PMC article. - Mapping brain structural differences and neuroreceptor correlates in Parkinson's disease visual hallucinations.
Vignando M, Ffytche D, Lewis SJG, Lee PH, Chung SJ, Weil RS, Hu MT, Mackay CE, Griffanti L, Pins D, Dujardin K, Jardri R, Taylor JP, Firbank M, McAlonan G, Mak HKF, Ho SL, Mehta MA. Vignando M, et al. Nat Commun. 2022 Jan 26;13(1):519. doi: 10.1038/s41467-022-28087-0. Nat Commun. 2022. PMID: 35082285 Free PMC article. - Menopausal hormone therapy and the female brain: leveraging neuroimaging and prescription registry data from the UK Biobank cohort.
Barth C, Galea LAM, Jacobs EG, Lee BH, Westlye LT, de Lange AG. Barth C, et al. medRxiv [Preprint]. 2025 Jan 14:2024.04.08.24305450. doi: 10.1101/2024.04.08.24305450. medRxiv. 2025. PMID: 38645009 Free PMC article. Preprint.
References
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300.
- Chen C-H, Pen Q, Schork AJ, Lo M-T, Fan C-C, Wang Y, Desikan RS, Bettella F, Pediatric Imaging. Neurocognition and Genetics Study. Alzheimer’s Disease Neuroimaging Initiative. Hagler DJ, Westlye LT, Kremen WS, Jernigan TL, Le Hellard S, Steen VM, Espeseth T, Huentelman M, Håberg AK, Agartz I, Djurovic S, Andreassen OE, Schork N, Dale AM. Large-scale genomics unveil polygenic architecture of human cortical surface area. Nat Commun. 2015;6:7549. doi: 10.1038/ncomms8549. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- The Disconnected Mind project/Age UK (GB)
- MR/R024065/1/MRC_/Medical Research Council/United Kingdom
- MR/K026992/1/MRC_/Medical Research Council/United Kingdom
- G0701120/MRC_/Medical Research Council/United Kingdom
- MR/N003403/1/MRC_/Medical Research Council/United Kingdom
- MR/M013111/1/MRC_/Medical Research Council/United Kingdom
- G1001245/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical