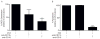Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating β2-integrin-dependent function in human eosinophils - PubMed (original) (raw)
Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating β2-integrin-dependent function in human eosinophils
Daniela J Carroll et al. J Allergy Clin Immunol. 2018 Jun.
Abstract
Background: Siglec-8 is a CD33 subfamily cell-surface receptor selectively expressed on human eosinophils. After cytokine priming, Siglec-8 mAb or glycan ligand binding causes eosinophil apoptosis associated with reactive oxygen species (ROS) production. Most CD33-related Siglecs function as inhibitory receptors, but the ability of Siglec-8 to stimulate eosinophil ROS production and apoptosis suggests that Siglec-8 might instead function as an activating receptor.
Objective: We sought to determine the role of IL-5 priming and identify the signaling molecules involved in Siglec-8 function for human eosinophils.
Methods: We used an mAb and/or a multimeric synthetic sulfated sialoglycan ligand recognizing Siglec-8 in combination with integrin blocking antibodies, pharmacologic inhibitors, phosphoproteomics, and Western blot analysis to define the necessity of various proteins involved in Siglec-8 function for human eosinophils.
Results: Cytokine priming was required to elicit the unanticipated finding that Siglec-8 engagement promotes rapid β2-integrin-dependent eosinophil adhesion. Also novel was the finding that this adhesion was necessary for subsequent ROS production and apoptosis. Siglec-8-mediated ROS was generated through reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation because pretreatment of eosinophils with catalase (an extracellular superoxide scavenger) or NSC 23766 (a Rac GTPase inhibitor) completely inhibited Siglec-8-mediated eosinophil apoptosis. Finally, engagement of Siglec-8 on IL-5-primed eosinophils resulted in increased phosphorylation of Akt, p38, and c-Jun N-terminal kinase 1 that was also β2-integrin dependent; pharmacologic inhibition of these kinases completely prevented Siglec-8-mediated eosinophil apoptosis.
Conclusions: These data demonstrate that Siglec-8 functions uniquely as an activating receptor on IL-5-primed eosinophils through a novel pathway involving regulation of β2-integrin-dependent adhesion, NADPH oxidase, and a subset of protein kinases.
Keywords: Akt; Eosinophil; Siglec-8; apoptosis; c-Jun N-terminal kinase (JNK); p38; phosphoproteomics; reduced nicotinamide adenine dinucleotide phosphate oxidase (NADPH); β(2)-integrin.
Copyright © 2017 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1. Siglec-8 antibody-induced changes in eosinophil morphology and phosphoproteomics in the presence or absence of IL-5 priming
(A) Freshly isolated or overnight IL-5 primed eosinophils were incubated with mAb 2C4 or IgG1 control for 1 h and adhesion and cell spreading was documented using light microscopy. Images are taken at 16× magnification and data are representative of three separate experiments with identical results. (B) Phosphorylated proteins were identified using mass spectrometry in lysates of IL-5 primed human eosinophils cultured with mAb 2C4 or IgG1 control for 15 min. Biological processes associated with Siglec-8 engagement on primary eosinophils were determined using functional enrichment analysis by GO terms as described in the Methods section. Data are from a single experiment and the numbers of identified phosphoproteins within each GO category are indicated.
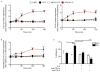
Figure 2. Siglec-8 engagement causes rapid CD11b/CD18 dependent eosinophil adhesion that requires IL-5 priming
(A–B) Freshly isolated or overnight IL-5 primed eosinophils were incubated with mAb 2C4 or IgG1 control and the surface expression of CD11b, CD18 and activated CD11b was assessed at various times for up to three hours. Levels of surface integrins were normalized to no treatment group (CD11b - GMFI was 1214 ± 130 in the presence of IL-5 priming and 1016 ± 312 in the absence of IL-5 priming; CD18 – GMFI was 3996 ± 697 in the presence of IL-5 priming and 3563 ± 399.8 in the absence of IL-5; activated CD11b – GMFI was 747.6 ± 196 in the presence of IL-5 priming and 626.2 ± 188.1 in the absence of IL-5 priming). (C) Freshly isolated or overnight IL-5 primed eosinophils were incubated with mAb 2C4 or IgG1 control for 2 h. Additionally, overnight IL-5 primed eosinophils were incubated with 10 μg/mL of CD11b or CD18 blocking antibody for 1 h as indicated before the addition of mAb 2C4 or IgG1 control for 2 h. After removal of non-adherent cells, percent eosinophil adhesion was determined. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 (n = 3–5; one-way ANOVA).
Figure 3. Siglec-8-mediated eosinophil ROS generation and apoptosis are β2-integrin dependent
(A) Eosinophils were incubated overnight with IL-5 then 10 μg/mL of CD11b or CD18 blocking antibody was added for 1 h before addition of mAb 2C4 or IgG1 control. ROS was assessed 1 h later and total ROS production was normalized to levels obtained with 2C4 mAb alone (GMFI 418 ± 97) following subtraction of background ROS levels (GMFI 146 ± 75). (B) Eosinophils were incubated as in panel A. Apoptosis was then assessed 18–24 h later and was normalized to levels of cell death seen with mAb 2C4 treatment alone (96 ± 0.13 % apoptosis) following subtraction of baseline apoptosis seen in the no treatment group (34 ± 5 % apoptosis). * p < 0.05; **** p < 0.0001 (n = 3 – 4, one-way ANOVA).
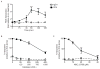
Figure 4. Siglec-8-mediated eosinophil apoptosis is inhibited by catalase and NSC 23766
(A) Eosinophils were incubated overnight with IL-5 before the addition of mAb 2C4 or IgG1 control, and total ROS levels were measured at various times for up to 3 h. Total ROS production was normalized to no treatment group (GMFI 79 ± 6) as in Figure 3. (B–C) Eosinophils were incubated overnight with IL-5 then catalase (superoxide scavenger), or NSC23766 (Rac GTPase inhibitor) was added for 1 h before the addition of mAb 2C4 or IgG1 control. Apoptosis was assessed 18–24 h later as in Figure 3. * p < 0.05; ** p < 0.01; **** p < 0.0001 (n = 3–4, one-way ANOVA).
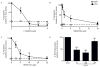
Figure 5. Siglec-8 engagement induces phosphorylation of various stress proteins in a β2-integrin dependent manner
(A) Eosinophils were incubated overnight with IL-5 before the addition of mAb 2C4 or IgG1 control for the indicated time points. Changes in phosphorylation of various stress proteins were measured using a phosphoprotein array. Shown are data from one experiment representative of at least two experiments. (B) Eosinophils were treated as in panel A and levels of selected phosphorylated intracellular proteins were measured by western blot at various times as indicated. Levels of total protein were used as loading controls. Shown are data from one experiment representative of at least three experiments. (C) Bar graphs show average quantified levels of phosphorylated proteins from replicate experiments. * p < 0.05; ** p < 0.01; *** p < 0.001 (n = 3–4, one-way ANOVA). (D) Eosinophils were incubated overnight with IL-5 then 10 μg/mL of CD18 blocking antibody was added for 1 h before addition of mAb 2C4 or IgG1 control for 1 h. Levels of selected phosphorylated intracellular proteins were measured by western blot. Levels of total protein were used as loading controls. Shown are data from one experiment representative of at least three experiments. (E) Bar graphs show average quantified levels of phosphorylated proteins from replicate experiments. * p < 0.05; ** p < 0.01; **** p < 0.0001 (n = 3, one-way ANOVA).
Figure 6. Siglec-8-mediated eosinophil apoptosis is inhibited by LY294002, SB203580 and SP600125
(A–C) Eosinophils were incubated overnight with IL-5 then indicated concentrations of LY294002 (PI3K/Akt inhibitor), SB203580 (p38 inhibitor) or SP600125 (JNK inhibitor) were added for 1 h before the addition of mAb 2C4 or IgG1 control. Apoptosis was assessed 18–24 h later as in Figure 3. (D) Eosinophils were incubated overnight with IL-5 then 1 μM LY294002 (PI3K/Akt inhibitor), 1.1 μM SB203580 (p38 inhibitor) or 4.3 μM SP600125 (JNK inhibitor) was added for 1 h before the addition of mAb 2C4 or IgG1 control. ROS was assessed one hour later as in figure 3. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 (n = 3–4, one-way ANOVA).
Figure 7. Schematic diagram of the proposed Siglec-8 signaling mechanism in IL-5 primed eosinophils
Siglec-8 engagement on IL-5 primed eosinophils (shown here with a Siglec-8 mAb but likely true of other Siglec-8-specific ligands) promotes CD11b/CD18 expression, activation and β2 integrin-dependent eosinophil adhesion, which is necessary for NADPH oxidase activation, ROS generation, activation of Akt, p38 and JNK1, and ultimately eosinophil apoptosis. Artwork by Jacqueline Schaffer
Similar articles
- Siglec-8 Signals Through a Non-Canonical Pathway to Cause Human Eosinophil Death In Vitro.
Carroll DJ, Cao Y, Bochner BS, O'Sullivan JA. Carroll DJ, et al. Front Immunol. 2021 Oct 11;12:737988. doi: 10.3389/fimmu.2021.737988. eCollection 2021. Front Immunol. 2021. PMID: 34721399 Free PMC article. - Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation.
Kano G, Almanan M, Bochner BS, Zimmermann N. Kano G, et al. J Allergy Clin Immunol. 2013 Aug;132(2):437-45. doi: 10.1016/j.jaci.2013.03.024. Epub 2013 May 16. J Allergy Clin Immunol. 2013. PMID: 23684072 Free PMC article. - Regulation of Siglec-8-induced intracellular reactive oxygen species production and eosinophil cell death by Src family kinases.
Kano G, Bochner BS, Zimmermann N. Kano G, et al. Immunobiology. 2017 Feb;222(2):343-349. doi: 10.1016/j.imbio.2016.09.006. Epub 2016 Sep 20. Immunobiology. 2017. PMID: 27682013 Free PMC article. - Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors.
Bochner BS. Bochner BS. Clin Exp Allergy. 2009 Mar;39(3):317-24. doi: 10.1111/j.1365-2222.2008.03173.x. Clin Exp Allergy. 2009. PMID: 19178537 Free PMC article. Review. - Discovery, Function, and Therapeutic Targeting of Siglec-8.
Youngblood BA, Leung J, Falahati R, Williams J, Schanin J, Brock EC, Singh B, Chang AT, O'Sullivan JA, Schleimer RP, Tomasevic N, Bebbington CR, Bochner BS. Youngblood BA, et al. Cells. 2020 Dec 24;10(1):19. doi: 10.3390/cells10010019. Cells. 2020. PMID: 33374255 Free PMC article. Review.
Cited by
- Sialic acid in the regulation of blood cell production, differentiation and turnover.
Irons EE, Gc S, Lau JTY. Irons EE, et al. Immunology. 2024 Aug;172(4):517-532. doi: 10.1111/imm.13780. Epub 2024 Mar 19. Immunology. 2024. PMID: 38503445 Review. - Interactions between Siglec-8 and endogenous sialylated cis ligands restrain cell death induction in human eosinophils and mast cells.
Cao Y, Rische CH, Bochner BS, O'Sullivan JA. Cao Y, et al. Front Immunol. 2023 Oct 20;14:1283370. doi: 10.3389/fimmu.2023.1283370. eCollection 2023. Front Immunol. 2023. PMID: 37928558 Free PMC article. - Siglecs as potential targets of therapy in human mast cell- and/or eosinophil-associated diseases.
O'Sullivan JA, Youngblood BA, Schleimer RP, Bochner BS. O'Sullivan JA, et al. Semin Immunol. 2023 Sep;69:101799. doi: 10.1016/j.smim.2023.101799. Epub 2023 Jul 4. Semin Immunol. 2023. PMID: 37413923 Free PMC article. Review. - Structures of the Inhibitory Receptor Siglec-8 in Complex with a High-Affinity Sialoside Analogue and a Therapeutic Antibody.
Lenza MP, Atxabal U, Nycholat C, Oyenarte I, Franconetti A, Quintana JI, Delgado S, Núñez-Franco R, Garnica Marroquín CT, Coelho H, Unione L, Jiménez-Oses G, Marcelo F, Schubert M, Paulson JC, Jiménez-Barbero J, Ereño-Orbea J. Lenza MP, et al. JACS Au. 2022 Dec 23;3(1):204-215. doi: 10.1021/jacsau.2c00592. eCollection 2023 Jan 23. JACS Au. 2022. PMID: 36711084 Free PMC article. - Discovery, classification, evolution and diversity of Siglecs.
Angata T, Varki A. Angata T, et al. Mol Aspects Med. 2023 Apr;90:101117. doi: 10.1016/j.mam.2022.101117. Epub 2022 Aug 18. Mol Aspects Med. 2023. PMID: 35989204 Free PMC article. Review.
References
- Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38:709–50. - PubMed
- Valent P. Pathogenesis, classification, and therapy of eosinophilia and eosinophil disorders. Blood Rev. 2009;23:157–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI072265/AI/NIAID NIH HHS/United States
- P01 HL107151/HL/NHLBI NIH HHS/United States
- P41 GM103490/GM/NIGMS NIH HHS/United States
- T32 AI083216/AI/NIAID NIH HHS/United States
- R01 AI072265/AI/NIAID NIH HHS/United States
- U19 AI136443/AI/NIAID NIH HHS/United States
- R01 AI041472/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous