Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice - PubMed (original) (raw)
Dietary Capsaicin Improves Glucose Homeostasis and Alters the Gut Microbiota in Obese Diabetic ob/ob Mice
Jun-Xian Song et al. Front Physiol. 2017.
Abstract
Background: The effects of capsaicin on obesity and glucose homeostasis are still controversial and the mechanisms underlying these effects remain largely unknown. This study aimed to investigate the potential relationship between the regulation of obesity and glucose homeostasis by dietary capsaicin and the alterations of gut microbiota in obese diabetic ob/ob mice. Methods: The ob/ob mice were subjected to a normal, low-capsaicin (0.01%), or high-capsaicin (0.02%) diet for 6 weeks, respectively. Obesity phenotypes, glucose homeostasis, the gut microbiota structure and composition, short-chain fatty acids, gastrointestinal hormones, and pro-inflammatory cytokines were measured. Results: Both the low- and high-capsaicin diets failed to prevent the increase in body weight, adiposity index, and Lee's obesity index. However, dietary capsaicin at both the low and high doses significantly inhibited the increase of fasting blood glucose and insulin levels. These inhibitory effects were comparable between the two groups. Similarly, dietary capsaicin resulted in remarkable improvement in glucose and insulin tolerance. In addition, neither the low- nor high-capsaicin diet could alter the α-diversity and β-diversity of the gut microbiota. Taxonomy-based analysis showed that both the low- and high-capsaicin diets, acting in similar ways, significantly increased the Firmicutes/Bacteroidetes ratio at the phylum level as well as increased the Roseburia abundance and decreased the Bacteroides and Parabacteroides abundances at the genus level. Spearman's correlation analysis revealed that the Roseburia abundance was negatively while the Bacteroides and Parabacteroides abundances were positively correlated to the fasting blood glucose level and area under the curve by the oral glucose tolerance test. Finally, the low- and high-capsaicin diets significantly increased the fecal butyrate and plasma total GLP-1 levels, but decreased plasma total ghrelin, TNF-α, IL-1β, and IL-6 levels as compared with the normal diet. Conclusions: The beneficial effects of dietary capsaicin on glucose homeostasis are likely associated with the alterations of specific bacteria at the genus level. These alterations in bacteria induced by dietary capsaicin contribute to improved glucose homeostasis through increasing short-chain fatty acids, regulating gastrointestinal hormones and inhibiting pro-inflammatory cytokines. However, our results should be interpreted cautiously due to the lower caloric intake at the initial stage after capsaicin diet administration.
Keywords: capsaicin; diabetes; glucose homeostasis; gut microbiota; obesity.
Figures
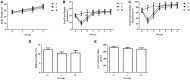
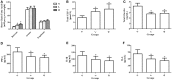
Figure 1
Effects of dietary capsaicin on obesity parameters. (A) Body weight; (B) total food intake, expressed as grams per mouse per week; (C) total caloric intake, expressed as kcal per mouse per week; (D) adiposity index, calculated according to the following formula: 100× (mesenteric fat weight + epididymal fat weight + perirenal fat weight)/body weight; (E) Lee's obesity index, calculated according to the following formula: body weight (g)0.33 × 104/naso-anal length (mm). N, normal diet group (n = 5); L, low-capsaicin diet group (n = 5); H, high-capsaicin diet group (n = 5). Data are shown as the mean ± SD; +P < 0.05, analyzed by one-way ANOVA with Tukey's post hoc test.
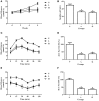
Figure 2
Effect of dietary capsaicin on glucose homeostasis. (A) Blood glucose; (B) insulin; (C) oral glucose tolerance test (OGTT); (D) area under the curve (AUC) for the OGTT, expressed as a percentage of the normal diet group (%); (E) insulin tolerance test (ITT); (F) area under the curve (AUC) for the ITT, expressed as a percentage of the normal diet group (%). N, normal diet group (n = 5); L, low-capsaicin diet group (n = 5); H, high-capsaicin diet group (n = 5). Data are shown as the mean ± SD; +P < 0.05, analyzed by one-way ANOVA with Tukey's post hoc test.
Figure 3
Rarefaction curves of observed species and Shannon-Wiener diversity for all samples. (A) Rarefaction curves of observed species from fecal samples of individual mice fed with a normal diet (red), low-capsaicin diet (green), or high-capsaicin diet (blue). (B) Rarefaction curves of Shannon-Wiener diversity from fecal samples of individual mice fed with a normal diet (red), low-capsaicin diet (green), or high-capsaicin diet (blue).
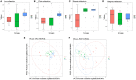
Figure 4
Effect of dietary capsaicin on the gut microbiota structure. (A) Ace estimator, (B) Chao estimator, (C) Shannon index, (D) Simpson index, (E) unweighted UniFrac distance-based principal coordinate analysis (PCoA), (F) weighted PCoA. N, normal diet group (n = 5); L, low-capsaicin diet group (n = 5); H, high-capsaicin diet group (n = 5). Data are shown as box and whisker plots. The box indicates the interquartile range (IQR, 75th to 25th percentiles of the data), and the mean value is shown as a line within the box; whiskers extend to the most extreme value within 1.5 × IQR, and outliers are shown as black dots. The results were analyzed by one-way ANOVA with Tukey's post hoc test or the Kruskal–Wallis test.
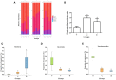
Figure 5
Effect of dietary capsaicin on the gut microbiota composition. (A) Changes in the taxonomic composition of the gut microbiota at the phylum level; (B) the Firmicutes/Bacteroidetes ratio; (C) the abundance of Roseburia; (D) the abundance of Bacteroides; (E) the abundance of Parabacteroides. N, normal diet group (n = 5); L, low-capsaicin diet group (n = 5); H, high-capsaicin diet group (n = 5). Data are shown as box and whisker plots. The box indicates the interquartile range (IQR, 75th to 25th percentiles of the data), and the mean value is shown as a line within the box; whiskers extend to the most extreme value within 1.5 × IQR, and outliers are shown as crosses. The results were analyzed by one-way ANOVA with Tukey's post hoc test or the Kruskal–Wallis test, +P < 0.05.
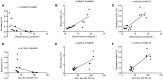
Figure 6
Correlations between the glucose parameters and the gut microbiota abundance. (A–C): Correlations of the blood glucose level with the abundance of Roseburia (A), Bacteroides (B), and Parabacteroides (C). (D–F) Correlations of the area under the curve (AUC) for the oral glucose tolerance test (OGTT) with the abundance of Roseburia (D), Bacteroides (E), and Parabacteroides (F). The data were analyzed by Spearman correlation analysis.
Figure 7
Effects of dietary capsaicin on short-chain fatty acids, gastrointestinal hormones, and pro-inflammatory cytokines. (A) Fecal short-chain fatty acid levels; (B) plasma total GLP-l level; (C) plasma total ghrelin level; (D) plasma TNF-α level; (E) plasma IL-1β level; (F) plasma IL-6 level. N, normal diet group (n = 5); L, low-capsaicin diet group (n = 5); H, high-capsaicin diet group (n = 5). Data are shown as the mean ± SD; +P < 0.05. The data analyzed by one-way ANOVA with Tukey's post hoc test.
Similar articles
- Gut microbiota and short chain fatty acids partially mediate the beneficial effects of inulin on metabolic disorders in obese ob/ob mice.
Guo J, Zhang M, Wang H, Li N, Lu Z, Li L, Hui S, Xu H. Guo J, et al. J Food Biochem. 2022 May;46(5):e14063. doi: 10.1111/jfbc.14063. Epub 2022 Feb 7. J Food Biochem. 2022. PMID: 35128673 - Dietary Epigallocatechin-3-Gallate Alters the Gut Microbiota of Obese Diabetic db/db Mice: Lactobacillus Is a Putative Target.
Park JM, Shin Y, Kim SH, Jin M, Choi JJ. Park JM, et al. J Med Food. 2020 Oct;23(10):1033-1042. doi: 10.1089/jmf.2020.4700. J Med Food. 2020. PMID: 33054538 - Dietary fat intake and age modulate the composition of the gut microbiota and colonic inflammation in C57BL/6J mice.
Kim SJ, Kim SE, Kim AR, Kang S, Park MY, Sung MK. Kim SJ, et al. BMC Microbiol. 2019 Aug 20;19(1):193. doi: 10.1186/s12866-019-1557-9. BMC Microbiol. 2019. PMID: 31429703 Free PMC article. - Gut decontamination with norfloxacin and ampicillin enhances insulin sensitivity in mice.
Chou CJ, Membrez M, Blancher F. Chou CJ, et al. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:127-37; discussion 137-40. doi: 10.1159/000146256. Nestle Nutr Workshop Ser Pediatr Program. 2008. PMID: 18626197 Review.
Cited by
- Plant-Derived (Poly)phenols and Their Metabolic Outcomes: The Pursuit of a Role for the Gut Microbiota.
Freitas PL, Miranda JPN, França LM, Paes AMA. Freitas PL, et al. Nutrients. 2022 Aug 26;14(17):3510. doi: 10.3390/nu14173510. Nutrients. 2022. PMID: 36079768 Free PMC article. Review. - TRPV1 Activation by Capsaicin Mediates Glucose Oxidation and ATP Production Independent of Insulin Signalling in Mouse Skeletal Muscle Cells.
Vahidi Ferdowsi P, Ahuja KDK, Beckett JM, Myers S. Vahidi Ferdowsi P, et al. Cells. 2021 Jun 21;10(6):1560. doi: 10.3390/cells10061560. Cells. 2021. PMID: 34205555 Free PMC article. - Capsaicin and Zinc Signalling Pathways as Promising Targets for Managing Insulin Resistance and Type 2 Diabetes.
Ferdowsi PV, Ahuja KDK, Beckett JM, Myers S. Ferdowsi PV, et al. Molecules. 2023 Mar 22;28(6):2861. doi: 10.3390/molecules28062861. Molecules. 2023. PMID: 36985831 Free PMC article. Review. - Gut Microbiota Mediates the Preventive Effects of Dietary Capsaicin Against Depression-Like Behavior Induced by Lipopolysaccharide in Mice.
Xia J, Gu L, Guo Y, Feng H, Chen S, Jurat J, Fu W, Zhang D. Xia J, et al. Front Cell Infect Microbiol. 2021 Apr 27;11:627608. doi: 10.3389/fcimb.2021.627608. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33987106 Free PMC article. - Involvement of TRPV1 Channels in Energy Homeostasis.
Christie S, Wittert GA, Li H, Page AJ. Christie S, et al. Front Endocrinol (Lausanne). 2018 Jul 31;9:420. doi: 10.3389/fendo.2018.00420. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30108548 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous