Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness - PubMed (original) (raw)
Review
Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness
Michael R Edwards et al. J Allergy Clin Immunol. 2017 Oct.
Abstract
Viral respiratory tract infections are associated with asthma inception in early life and asthma exacerbations in older children and adults. Although how viruses influence asthma inception is poorly understood, much research has focused on the host response to respiratory viruses and how viruses can promote; or how the host response is affected by subsequent allergen sensitization and exposure. This review focuses on the innate interferon-mediated host response to respiratory viruses and discusses and summarizes the available evidence that this response is impaired or suboptimal. In addition, the ability of respiratory viruses to act in a synergistic or additive manner with TH2 pathways will be discussed. In this review we argue that these 2 outcomes are likely linked and discuss the available evidence that shows reciprocal negative regulation between innate interferons and TH2 mediators. With the renewed interest in anti-TH2 biologics, we propose a rationale for why they are particularly successful in controlling asthma exacerbations and suggest ways in which future clinical studies could be used to find direct evidence for this hypothesis.
Keywords: T(H)2; Virus; allergic inflammation; asthma; interferon.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
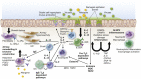
Fig 1
Overview of additive or synergistic effects of allergen exposure and viral infection driving both pro-TH2 and IgE/TH2 inflammation. Viral infection damages the epithelial barrier and results in the pro-TH2 cytokines IL-33, IL-25, and thymic stromal lymphopoietin. These cytokines act on ILC2s, DCs, and TH2 cells, resulting in production of the TH2 cytokines IL-13, IL-4, and IL-5. These cytokines have a central role in allergic asthma: IL-4 and IL-13 drive antibody class-switching to IgE in B cells, IL-13 can act also on airway smooth muscle cells, causing bronchoconstriction and contributing to airway remodeling, and IL-5 contributes to eosinophil production. Actions of IL-4 and IL-13 and viruses on airway epithelial cells can induce the eotaxins that attract eosinophils and the chemokines macrophage-derived chemokine (MDC) and thymus and activation-regulated chemokine (TARC), which attract TH2 cells into the airway. IgE cross-linking by allergen on mast cells produces histamine, leukotrienes, and the prostaglandins PGD2 and PGE2, which further promote bronchoconstriction. PGD2 binds chemoattractant receptor-homologous molecule expressed on TH2 cells (CRTH2) and activates basophils, TH2 cells, and ILC2s. Viruses can also cause oxidative stress, and pathogen-associated molecular pattern (PAMP) and damage-associated molecular pattern (DAMP) production can lead to the proinflammatory cytokines IL-1α/β, TNF, and IL-6. This generally results in neutrophilic inflammation and activation of macrophages. Allergen-induced IL-1α can also promote the pro-TH2 response and lead to further IL-33 production and activation of ILC2s. ENA-78, Epithelial-derived neutrophil-activating protein 78; GRO-α, melanoma growth stimulating activity-α; IL-4R, IL-4 receptor; IL-13R, IL-13 receptor; IL-25R, IL-25 receptor; IL-33R, IL-33 receptor.
Fig 2
Summary of mechanisms of reciprocal negative regulation of type I and III interferons and TH2 pathways in asthmatic patients. TH2 cytokines can negatively regulate virus-induced interferons; cross-linking of cell-bound IgE on DCs prevents virus-induced IFN-α on infection; pretreatment of HBECs with IL-4, IL-13, or TGF-β results in low IFN-β and IL-29 induction on infection; and IL-33 can act on DCs and reduce IRAK levels and hence IFN-α induction on infection. Interferons can suppress TH2 responses; pretreatment of T cells with IFN-α/β reduces GATA-3 and IL-5 levels in TH2 cells; viruses or TLR7 agonists produce IFN-α/β from DCs, which act on TH2 cells to reduce level of IL-4, IL-5, and IL-13; and IFN-β pretreatment of ILC2s causes a reduction in IL-4, IL-5, and IL-13. Finally, IL-28A (IFN-λ) acts on DCs to reduce OX40 ligand and prevent TH2 cell expression of IL-5 and IL-13. BECs, Bronchial epithelial cell; pDCs, plasmacytoid dendritic cells.
Comment in
- Viral induced overproduction of epithelial TSLP: Role in exacerbations of asthma and COPD?
Uller L, Persson C. Uller L, et al. J Allergy Clin Immunol. 2018 Aug;142(2):712. doi: 10.1016/j.jaci.2018.01.051. Epub 2018 Apr 5. J Allergy Clin Immunol. 2018. PMID: 29628119 No abstract available.
Similar articles
- Immune mechanisms of respiratory viral infections in asthma.
Farne HA, Johnston SL. Farne HA, et al. Curr Opin Immunol. 2017 Oct;48:31-37. doi: 10.1016/j.coi.2017.07.017. Epub 2017 Aug 14. Curr Opin Immunol. 2017. PMID: 28818789 Review. - The sentinel role of the airway epithelium in asthma pathogenesis.
Holgate ST. Holgate ST. Immunol Rev. 2011 Jul;242(1):205-19. doi: 10.1111/j.1600-065X.2011.01030.x. Immunol Rev. 2011. PMID: 21682747 Review. - Respiratory viral infections as promoters of allergic sensitization and asthma in animal models.
Schwarze J, Gelfand EW. Schwarze J, et al. Eur Respir J. 2002 Feb;19(2):341-9. doi: 10.1183/09031936.02.00254302. Eur Respir J. 2002. PMID: 11866016 - New perspectives on mechanisms underlying chronic allergic inflammation and asthma in 2007.
Broide D. Broide D. J Allergy Clin Immunol. 2008 Sep;122(3):475-80. doi: 10.1016/j.jaci.2008.06.025. Epub 2008 Aug 9. J Allergy Clin Immunol. 2008. PMID: 18694589 Review. - Respiratory viral infection, epithelial cytokines, and innate lymphoid cells in asthma exacerbations.
Kumar RK, Foster PS, Rosenberg HF. Kumar RK, et al. J Leukoc Biol. 2014 Sep;96(3):391-6. doi: 10.1189/jlb.3RI0314-129R. Epub 2014 Jun 5. J Leukoc Biol. 2014. PMID: 24904000 Free PMC article. Review.
Cited by
- Insights Into Type I and III Interferons in Asthma and Exacerbations.
Rich HE, Antos D, Melton NR, Alcorn JF, Manni ML. Rich HE, et al. Front Immunol. 2020 Sep 25;11:574027. doi: 10.3389/fimmu.2020.574027. eCollection 2020. Front Immunol. 2020. PMID: 33101299 Free PMC article. Review. - House dust mite sensitization and exposure affects bronchial epithelial anti-microbial response to viral stimuli in patients with asthma.
Cerps S, Sverrild A, Ramu S, Nieto-Fontarigo JJ, Akbarshahi H, Menzel M, Andersson C, Tillgren S, Hvidtfeldt M, Porsbjerg C, Uller L. Cerps S, et al. Allergy. 2022 Aug;77(8):2498-2508. doi: 10.1111/all.15243. Epub 2022 Feb 11. Allergy. 2022. PMID: 35114024 Free PMC article. - Environmental exposures and mechanisms in allergy and asthma development.
Murrison LB, Brandt EB, Myers JB, Hershey GKK. Murrison LB, et al. J Clin Invest. 2019 Apr 1;129(4):1504-1515. doi: 10.1172/JCI124612. Epub 2019 Feb 11. J Clin Invest. 2019. PMID: 30741719 Free PMC article. Review. - Considerations on biologicals for patients with allergic disease in times of the COVID-19 pandemic: An EAACI statement.
Vultaggio A, Agache I, Akdis CA, Akdis M, Bavbek S, Bossios A, Bousquet J, Boyman O, Chaker AM, Chan S, Chatzipetrou A, Feleszko W, Firinu D, Jutel M, Kauppi P, Klimek L, Kolios A, Kothari A, Kowalski ML, Matucci A, Palomares O, Pfaar O, Rogala B, Untersmayr E, Eiwegger T. Vultaggio A, et al. Allergy. 2020 Nov;75(11):2764-2774. doi: 10.1111/all.14407. Allergy. 2020. PMID: 32500526 Free PMC article. - Host-microbiota interactions in immune-mediated diseases.
Ruff WE, Greiling TM, Kriegel MA. Ruff WE, et al. Nat Rev Microbiol. 2020 Sep;18(9):521-538. doi: 10.1038/s41579-020-0367-2. Epub 2020 May 26. Nat Rev Microbiol. 2020. PMID: 32457482 Review.
References
- Bufe A., Gehlhar K., Grage-Griebenow E., Ernst M. Atopic phenotype in children is associated with decreased virus-induced interferon-alpha release. Int Arch Allergy Immunol. 2002;127:82–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical