LAMTOR/Ragulator is a negative regulator of Arl8b- and BORC-dependent late endosomal positioning - PubMed (original) (raw)
. 2017 Dec 4;216(12):4199-4215.
doi: 10.1083/jcb.201703061. Epub 2017 Oct 9.
Mariana E G de Araujo 1, Georg F Vogel 1 2, Cedric H De Smet 1, Daniela Eberharter 1, Manuele Rebsamen 3, Elena L Rudashevskaya 3 4, Leopold Kremser 5, Teodor Yordanov 1, Philipp Tschaikner 6, Barbara G Fürnrohr 7, Stefan Lechner 7, Theresia Dunzendorfer-Matt 7, Klaus Scheffzek 7, Keiryn L Bennett 3, Giulio Superti-Furga 3 8, Herbert H Lindner 5, Taras Stasyk 9, Lukas A Huber 10
Affiliations
- PMID: 28993467
- PMCID: PMC5716276
- DOI: 10.1083/jcb.201703061
LAMTOR/Ragulator is a negative regulator of Arl8b- and BORC-dependent late endosomal positioning
Przemyslaw A Filipek et al. J Cell Biol. 2017.
Abstract
Signaling from lysosomes controls cellular clearance and energy metabolism. Lysosomal malfunction has been implicated in several pathologies, including neurodegeneration, cancer, infection, immunodeficiency, and obesity. Interestingly, many functions are dependent on the organelle position. Lysosomal motility requires the integration of extracellular and intracellular signals that converge on a competition between motor proteins that ultimately control lysosomal movement on microtubules. Here, we identify a novel upstream control mechanism of Arl8b-dependent lysosomal movement toward the periphery of the cell. We show that the C-terminal domain of lyspersin, a subunit of BLOC-1-related complex (BORC), is essential and sufficient for BORC-dependent recruitment of Arl8b to lysosomes. In addition, we establish lyspersin as the linker between BORC and late endosomal/lysosomal adaptor and mitogen activated protein kinase and mechanistic target of rapamycin activator (LAMTOR) complexes and show that epidermal growth factor stimulation decreases LAMTOR/BORC association, thereby promoting BORC- and Arl8b-dependent lysosomal centrifugal transport.
© 2017 Filipek et al.
Figures
Figure 1.
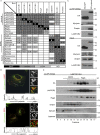
The LAMTOR pentamer participates in two distinct multimeric complexes on late endosomes/lysosomes. (A) Interactome analysis identifies two LAMTOR populations associating either with RagA/C, SLC38A9, and Raptor or with BORC. HS-tagged baits (LAMTOR1, LAMTOR3, LAMTOR4, LAMTOR5, RagA, RagC, lyspersin, BLOS1, and pallidin) were purified by TAP and analyzed by LC-MS/MS. The normalized abundances (% of total spectral counts [for LAMTOR 1, 3, 4, and 5, RagA, RagC, and BLOS1] or peak areas [for lyspersin and pallidin]) of the interacting partners are shown (Table S1). BAIT (black), bait; HIGH (dark gray), high abundance interactors (≥2%); LOW (bright gray), low abundance interactors (<2%); ND (white), no interaction detected. (B) HA-lyspersin partially colocalizes with late endosomes/lysosomes (LAMP1) but not with early endosomes (EEA1). HeLa cells were transfected with HA-lyspersin. Single plane confocal images of indirect IF of HA-lyspersin (green) and endogenous endosomal markers (red) are shown. Intensity profiles for HA-lyspersin (green) and LAMP1 or EEA1 (red) along the cross section lines are shown. Representative images are shown. Bars: 10 µm; (insets) 1 µm. (C) Immunoblotting analysis of HS-lyspersin Strep-purified complexes confirms the interaction of lyspersin with myrlysin, snapin, and LAMTOR2. (D) The majority of the pentameric LAMTOR complex associates with RagA. Only a minor fraction interacts with BORC components. The eluate from Strep-purified LAMTOR1-SH was subjected to size exclusion chromatography and analyzed by WB with the indicated antibodies.
Figure 2.
The LAMTOR–BORC interaction is lyspersin-dependent. (A) Deletion of lyspersin abolishes the interaction between LAMTOR and the remaining BORC subunits (BORCΔlyspersin). In contrast, the LAMTOR-RagA interaction remains unaffected. HA immunoprecipitates from control, WT, and lyspersin KO HeLa cells stably expressing LAMTOR1-HA were analyzed by immunoblotting. (B) Annotated representation of lyspersin truncation mutants. N-domain, N-terminal region; C-domain, C-terminal region. (C) Lyspersin interacts with the LAMTOR complex through its PRR whereas the C-domain of lyspersin is responsible for the interaction with the remaining BORC subunits. The DUF2365 domain is sufficient to mediate the interaction with both LAMTOR and BORCΔlyspersin. Immunoblotting analysis of Strep coprecipitates from lyspersin truncation mutants inducibly expressed in HEK293 Flp-In T-REx cells.
Figure 3.
The direct inter- and intramolecular interactions of LAMTOR and BORCΔBLOC-1 detected by in vitro chemical cross-linking. (A) Cross-linking of in vitro copurified LAMTOR (LAMTOR1Δ1-20, LAMTOR2, LAMTOR3, LAMTOR4, and LAMTOR5) and BORCΔBLOC-1 (lyspersin, myrlysin, KxDL1, MEF2BNB, and diaskedin) complexes with 0.2 mM BS3 results in a higher molecular weight band (arrow) detected by anti-lyspersin and anti-LAMTOR2 antibodies. (B) List of unique cross-links identified by mass spectrometry. Recombinant protein complexes were purified and cross-linked as described in A. The cross-linked proteins were digested and the tryptic peptides analyzed by mass spectrometry. Annotated are the cross-linked proteins, relevant peptide sequences, the lysines being cross-linked and the total number of identified peptide spectra matched (PSM). (C) The N terminus of LAMTOR2 cross-links with K224 of lyspersin (closest lysine to PRR); myrlysin and KxDL1 cross-link with the C-domain of lyspersin. The thickness of the lanes in the graphical representation corresponds to the number of cross-links identified in B.
Figure 4.
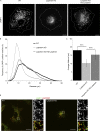
Lyspersin regulates endosomal positioning. (A) Deletion of lyspersin results in the accumulation of late endosomes/lysosomes in the perinuclear region, and the distribution can be restored by reconstitution of the lyspersin KO cells with a tagged version of the protein. Indirect IF images of HeLa WT, lyspersin KO, and rescue cell line. Representative single-channel images of endogenous lysosomal marker LAMP1 are shown. Bars, 10 µm. (B) Deletion of lyspersin leads to an increase in the number of perinuclear LAMP1-positive endosomes. Images were taken on a confocal microscope and analyzed using Huygens and Imaris software. Number of cells per genotype, n ≥ 15. Depicted is the percentage of late endosomes found at a given distance from the center of nucleus. (C) The mean distance of late endosomes/lysosomes to the center of nucleus is significantly smaller in lyspersin KO cells than in WT or rescued cells. Images were analyzed as in B. Mean ± SD. ***, P ≤ 0.001. n ≥ 15 cells. WT/lyspersin KO, P = 3.0 × 10-12; lyspersin KO HS-lyspersin/lyspersin KO, P = 1.2 × 10-6. (D) LAMTOR1 localizes to late endosomes in a lyspersin independent manner. Control and lyspersin depleted cells were analyzed. Shown are representative, single plane confocal images of indirect IF of endogenous LAMTOR1 (red) and endogenous LAMP1 (green). Bars: 10 µm; (inset) 1 µm.
Figure 5.
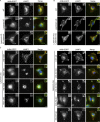
Arl8b requires lyspersin but not LAMTOR for late endosomal/lysosomal localization. (A) Lyspersin is essential to recruit Arl8b to LAMP1-positive endosomes. Control WT, lyspersin KO, and rescue cell lines were transfected with Arl8b-EGFP. Representative, merged, and single-channel images of endogenous LAMP1 (red) and Arl8b-EGFP (green) are shown. Bars: 10 µm; inset, 2 µm. (B) Arl8b recruitment to late endosomes/lysosomes is LAMTOR-independent. Control WT, LAMTOR1 KO, and rescue cell lines were transfected with Arl8b-EGFP. Experiment was performed as in A. Representative images are shown. Bars: 10 µm; inset, 2 µm. (C) The C-domain of lyspersin or truncation mutants containing the C-domain are sufficient to restore the recruitment of Arl8b to LAMP1-positive endosomes in lyspersin KO cells. Lyspersin KO cells were cotransfected with Arl8b-EGFP and truncation mutants of lyspersin (Fig. 2 B). Experiment was performed as in A. Representative images are shown. Bars: 10 µm; (inset) 2 µm.
Figure 6.
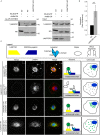
LAMTOR controls endosomal positioning by negatively regulating the BORC–Arl8b interaction. (A) The interaction of Arl8b-SH with the BORC subunit lyspersin is increased in LAMTOR1 KO cells. Strep-purified Arl8b-SH or HS-EGFP from LAMTOR1 KO and WT cells were analyzed by immunoblotting. (B) Results of four biologically independent experiments were quantified. Mean ± SD. **, P ≤ 0.01; P = 0.0059. (C) In lyspersin KO cells, the irreversible association of BORC with LAMTOR, achieved by Venus fluorophore reconstitution (lyspersin-VF1, LAMTOR1-VF2), immobilizes LAMP1-positive endosomes in the perinuclear region. No interaction (Venus signal) was detected in cells expressing lyspersin-VF1 and the control Zipper-VF2. Lyspersin-VF1 expression in lyspersin KO cells restores transportation of late endosomes toward the cell periphery. The overexpression of two LAMTOR subunits (LAMTOR3-VF1 and LAMTOR1-FV2) results in Venus-fluorophore reconstitution, indicating an interaction between both proteins in lyspersin KO and WT cells. The Venus signal in peripheral LAMP1 vesicles is found exclusively in WT cells. Analysis of Venus-fluorophore reconstitution (green) and indirect IF of endogenous LAMP1 (red). Representative images are shown. Arrows indicate endosomes positive for LAMP1 and BORC–LAMTOR irreversible interaction (Venus). Arrowheads show LAMP1-positive but BORC–LAMTOR interaction–negative late endosomes. Dashed line indicates cell borders. Representative images are shown. Bars: 10 µm; (inset) 2 µm. Legend: yellow trapezoid, LAMTOR complex; dark blue trapezoid, BORCΔlyspersin; light blue shape with discrimination of individual domains (N-domain, PRR, and C-domain), lyspersin; white ellipse, Zipper (negative control); white semicircles, VFs; two green semicircles together, Venus reconstitution and interaction between tagged proteins. The adjacent cell model depicts the presence (green) or absence of interaction (gray) and the overall position of late endosomes in the transfected cells.
Figure 7.
EGF regulates LAMTOR–BORC interaction. (A) The interaction between BORC and LAMTOR complexes is decreased upon EGF stimulation. Lyspersin KO cells stably expressing HS-lyspersin were starved for FBS and stimulated with 100 ng/ml EGF for 1 h. Obtained lysates were Strep-purified and analyzed by immunoblotting. EGFR degradation was used as control for the treatment. (B) Quantification of the results of three biologically independent experiments. Mean ± SD. ***, P ≤ 0.001; P = 0.000352. (C) Confirmation that the interaction between BORC and LAMTOR complexes is decreased upon EGF stimulation with HEK293 Flp-In T-REx cells inducibly expressing diaskedin-SH starved and stimulated as in A. Immunoblotting analysis of the Strep-purified complexes. EGFR degradation was used as control for the treatment. (D) Quantification of the results of three biologically independent experiments. Mean ± SD. ***, P ≤ 0.001; P = 0.000938. (E) Arbitrary cutoff for peripheral late endosomes based on LAMP1-positive vesicles distribution in starved WT HeLa cells. Epifluorescence images were analyzed using ImageJ software with our macro RadialIntensityProfile (Materials and methods). Depicted is the signal intensity of LAMP1 found at the indicated distance from the center of nucleus, perinuclear lysosomes ≤16 µm, peripheral lysosomes ≥16 µm. Number of cells per genotype, n ≥ 50 cells. (F) Frequency of peripheral lysosomes increases upon EGF stimulation. WT HeLa cells were starved for FBS overnight and stimulated with EGF for the indicated time points and subsequently treated as in E. Depicted is the signal intensity of LAMP1 found at ≥16 µm from the center of the nucleus. Mean ± SD. Not significant (n.s.), P > 0.05; ****, P ≤ 0.0001 (0 h/1 h P = 0.630, 0 h/4 h P = 1.1 × 10-8, 0 h/8 h P = 3.2 × 10-7). Number of cells per genotype, n ≥ 50 cells.
Figure 8.
Model. In control cells, two mutually exclusive, synchronous events take place on late endosomes/lysosomes. On the one hand, the BORC–LAMTOR interaction blocks recruitment of Arl8b and leads to the perinuclear position of late endosomes. On the other hand, the BORC–Arl8b interaction promotes the transport of late endosomes, resulting in a peripheral distribution. The steady-state scenario is a combination of both, with some late endosomes being transported toward the cell periphery, whereas others remain perinuclear. In lyspersin KO cells, the remaining subunits of BORC (BORCΔlyspersin) are insufficient to recruit Arl8b to late endosomes, resulting in perinuclear clustering of the organelles. Lyspersin depletion also prevents LAMTOR–BORCΔlyspersin interaction. Depletion of the LAMTOR complex increases BORC–Arl8b interaction, thereby promoting transport and peripheral accumulation of LAMP1-positive endosomes. Yellow trapezoid, LAMTOR complex; dark blue trapezoid, BORCΔlyspersin; light blue shape, lyspersin with discrimination of individual domains (N-domain, PRR, and C-domain); red shape, Arl8b.
Comment in
- Ragulator-a multifaceted regulator of lysosomal signaling and trafficking.
Colaço A, Jäättelä M. Colaço A, et al. J Cell Biol. 2017 Dec 4;216(12):3895-3898. doi: 10.1083/jcb.201710039. Epub 2017 Nov 14. J Cell Biol. 2017. PMID: 29138253 Free PMC article.
Similar articles
- A Ragulator-BORC interaction controls lysosome positioning in response to amino acid availability.
Pu J, Keren-Kaplan T, Bonifacino JS. Pu J, et al. J Cell Biol. 2017 Dec 4;216(12):4183-4197. doi: 10.1083/jcb.201703094. Epub 2017 Oct 9. J Cell Biol. 2017. PMID: 28993468 Free PMC article. - BORC, a multisubunit complex that regulates lysosome positioning.
Pu J, Schindler C, Jia R, Jarnik M, Backlund P, Bonifacino JS. Pu J, et al. Dev Cell. 2015 Apr 20;33(2):176-88. doi: 10.1016/j.devcel.2015.02.011. Dev Cell. 2015. PMID: 25898167 Free PMC article. - Biogenesis of lysosome-related organelles complex-1 (BORC) regulates late endosomal/lysosomal size through PIKfyve-dependent phosphatidylinositol-3,5-bisphosphate.
Yordanov TE, Hipolito VEB, Liebscher G, Vogel GF, Stasyk T, Herrmann C, Geley S, Teis D, Botelho RJ, Hess MW, Huber LA. Yordanov TE, et al. Traffic. 2019 Sep;20(9):674-696. doi: 10.1111/tra.12679. Traffic. 2019. PMID: 31314175 Free PMC article. - Neurodevelopmental disease mechanisms, primary cilia, and endosomes converge on the BLOC-1 and BORC complexes.
Hartwig C, Monis WJ, Chen X, Dickman DK, Pazour GJ, Faundez V. Hartwig C, et al. Dev Neurobiol. 2018 Mar;78(3):311-330. doi: 10.1002/dneu.22542. Epub 2017 Oct 13. Dev Neurobiol. 2018. PMID: 28986965 Free PMC article. Review. - Endolysosomal compartments as platforms for orchestrating innate immune and metabolic sensors.
Miyake K, Saitoh SI, Sato R, Shibata T, Fukui R, Murakami Y. Miyake K, et al. J Leukoc Biol. 2019 Oct;106(4):853-862. doi: 10.1002/JLB.MR0119-020R. Epub 2019 Jun 20. J Leukoc Biol. 2019. PMID: 31219657 Review.
Cited by
- Molecular Mechanisms of Lysosome and Nucleus Communication.
Zhao Q, Gao SM, Wang MC. Zhao Q, et al. Trends Biochem Sci. 2020 Nov;45(11):978-991. doi: 10.1016/j.tibs.2020.06.004. Epub 2020 Jul 2. Trends Biochem Sci. 2020. PMID: 32624271 Free PMC article. Review. - Targeting lysosome function causes selective cytotoxicity in VHL-inactivated renal cell carcinomas.
Bouhamdani N, Comeau D, Coholan A, Cormier K, Turcotte S. Bouhamdani N, et al. Carcinogenesis. 2020 Jul 10;41(6):828-840. doi: 10.1093/carcin/bgz161. Carcinogenesis. 2020. PMID: 31556451 Free PMC article. - Lysosomes as dynamic regulators of cell and organismal homeostasis.
Ballabio A, Bonifacino JS. Ballabio A, et al. Nat Rev Mol Cell Biol. 2020 Feb;21(2):101-118. doi: 10.1038/s41580-019-0185-4. Epub 2019 Nov 25. Nat Rev Mol Cell Biol. 2020. PMID: 31768005 Review. - The TMEM127 human tumor suppressor is a component of the mTORC1 lysosomal nutrient-sensing complex.
Deng Y, Qin Y, Srikantan S, Luo A, Cheng ZM, Flores SK, Vogel KS, Wang E, Dahia PLM. Deng Y, et al. Hum Mol Genet. 2018 May 15;27(10):1794-1808. doi: 10.1093/hmg/ddy095. Hum Mol Genet. 2018. PMID: 29547888 Free PMC article. - Lysosome Positioning Influences mTORC2 and AKT Signaling.
Jia R, Bonifacino JS. Jia R, et al. Mol Cell. 2019 Jul 11;75(1):26-38.e3. doi: 10.1016/j.molcel.2019.05.009. Epub 2019 May 23. Mol Cell. 2019. PMID: 31130364 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials