Age Drives Distortion of Brain Metabolic, Vascular and Cognitive Functions, and the Gut Microbiome - PubMed (original) (raw)
Age Drives Distortion of Brain Metabolic, Vascular and Cognitive Functions, and the Gut Microbiome
Jared D Hoffman et al. Front Aging Neurosci. 2017.
Abstract
Advancing age is the top risk factor for the development of neurodegenerative disorders, including Alzheimer's disease (AD). However, the contribution of aging processes to AD etiology remains unclear. Emerging evidence shows that reduced brain metabolic and vascular functions occur decades before the onset of cognitive impairments, and these reductions are highly associated with low-grade, chronic inflammation developed in the brain over time. Interestingly, recent findings suggest that the gut microbiota may also play a critical role in modulating immune responses in the brain via the brain-gut axis. In this study, our goal was to identify associations between deleterious changes in brain metabolism, cerebral blood flow (CBF), gut microbiome and cognition in aging, and potential implications for AD development. We conducted our study with a group of young mice (5-6 months of age) and compared those to old mice (18-20 months of age) by utilizing metabolic profiling, neuroimaging, gut microbiome analysis, behavioral assessments and biochemical assays. We found that compared to young mice, old mice had significantly increased levels of numerous amino acids and fatty acids that are highly associated with inflammation and AD biomarkers. In the gut microbiome analyses, we found that old mice had increased Firmicutes/Bacteroidetes ratio and alpha diversity. We also found impaired blood-brain barrier (BBB) function and reduced CBF as well as compromised learning and memory and increased anxiety, clinical symptoms often seen in AD patients, in old mice. Our study suggests that the aging process involves deleterious changes in brain metabolic, vascular and cognitive functions, and gut microbiome structure and diversity, all which may lead to inflammation and thus increase the risk for AD. Future studies conducting comprehensive and integrative characterization of brain aging, including crosstalk with peripheral systems and factors, will be necessary to define the mechanisms underlying the shift from normal aging to pathological processes in the etiology of AD.
Keywords: Alzheimer’s disease; MRI; aging; anxiety; brain metabolism; cognition; gut microbiome; neurovascular function.
Figures
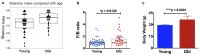
Figure 1
(A) The old mice showed a higher alpha-diversity, as indicated by the Shannon index, than the young mice (p = 0.02). Compared with the young mice, the old mice had significantly increased (B) Firmicutes/Bacteroidetes ratio and (C) body weight. N = 39 and 28 for young and old mice, respectively. Data are presented as mean ± SEM.
Figure 2
(A) The old mice had a significant increase in inducible nitric oxide synthase (iNOS) in the brain compared to the young mice (N = 7–8 per group). The old mice had acute elevations (2 h after feeding) in (B) 3-indoxyl sulfate and (C) phenol sulfate; those levels returned to baseline 4 h later (N = 6 and 4 for young and old mice, respectively).
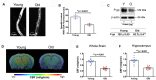
Figure 3
(A) Representative confocal images showing decreased luminal accumulation of N-ε(4-nitro-benzofurazan-7-yl)-D-Lys(8)-cyclosporin A (NBD-CSA) fluorescence (white) in brain capillaries isolated from the old mice compared to young mice, indicating reduced P-glycoprotein (P-gp) activity. (B) Corresponding quantitative fluorescence data; images are shown in arbitrary fluorescence units (scale 0–255). Data are mean ± SEM for 10 capillaries from one preparation of 10 mice. (C) Western blotting (WB) for P-gp from the cortical vasculature, β-Actin was used as loading control (top); corresponding values are shown in the table (bottom). The WB data from the old mice were normalized to β-Actin and compared to the young mice (100%), **p < 0.01. (D) Cerebral blood flow (CBF) maps superimposed on structural images; the color code indicates the level of CBF on a linear scale. Quantitative CBF (ml/g/min) obtained from (E) the whole brain and (F) hippocampus (N = 12 per group). Data are mean ± SEM.
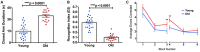
Figure 4
(A) The elevated plus maze (EPM) found the old mice to have a significantly higher closed arm duration compared to the young group. (B) The Novel Object Recognition (NOR) test found the old group had a significantly lower recognition index, or D2, than the young group. (C) The average errors made by the young and old mice during the Radial Arm Water Maze (RAWM) split into six blocks. The significant difference between the two groups in average errors corrected showing in Block 3 (p = 0.0307) and Block 4 (p = 0.0045). N = 22 and 18 for young and old mice, respectively. Data are presented as mean ± SEM. **p < 0.05.
Figure 5
Proposed associations of age-dependent changes in brain metabolism, vascular integrity, gut microbiome, cognition and anxiety level. Inflammation in aging, or inflammaging, might play a critical role in be a driving the deleterious changes in the brain and gut.
Similar articles
- Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer's disease.
Cui B, Su D, Li W, She X, Zhang M, Wang R, Zhai Q. Cui B, et al. J Neuroinflammation. 2018 Jun 22;15(1):190. doi: 10.1186/s12974-018-1223-4. J Neuroinflammation. 2018. PMID: 29933742 Free PMC article. - The Microbiota-Gut-Brain Axis in Alzheimer's Disease: A Review of Taxonomic Alterations and Potential Avenues for Interventions.
Murray ER, Kemp M, Nguyen TT. Murray ER, et al. Arch Clin Neuropsychol. 2022 Feb 22;37(3):595-607. doi: 10.1093/arclin/acac008. Arch Clin Neuropsychol. 2022. PMID: 35202456 Free PMC article. Review. - Mannan oligosaccharide attenuates cognitive and behavioral disorders in the 5xFAD Alzheimer's disease mouse model via regulating the gut microbiota-brain axis.
Liu Q, Xi Y, Wang Q, Liu J, Li P, Meng X, Liu K, Chen W, Liu X, Liu Z. Liu Q, et al. Brain Behav Immun. 2021 Jul;95:330-343. doi: 10.1016/j.bbi.2021.04.005. Epub 2021 Apr 9. Brain Behav Immun. 2021. PMID: 33839232 - Ketogenic diet enhances neurovascular function with altered gut microbiome in young healthy mice.
Ma D, Wang AC, Parikh I, Green SJ, Hoffman JD, Chlipala G, Murphy MP, Sokola BS, Bauer B, Hartz AMS, Lin AL. Ma D, et al. Sci Rep. 2018 Apr 27;8(1):6670. doi: 10.1038/s41598-018-25190-5. Sci Rep. 2018. PMID: 29703936 Free PMC article. - A Review of the Brain-Gut-Microbiome Axis and the Potential Role of Microbiota in Alzheimer's Disease.
Sun M, Ma K, Wen J, Wang G, Zhang C, Li Q, Bao X, Wang H. Sun M, et al. J Alzheimers Dis. 2020;73(3):849-865. doi: 10.3233/JAD-190872. J Alzheimers Dis. 2020. PMID: 31884474 Review.
Cited by
- Targeting the blood-brain barrier to delay aging-accompanied neurological diseases by modulating gut microbiota, circadian rhythms, and their interplays.
Wang Y, Du W, Hu X, Yu X, Guo C, Jin X, Wang W. Wang Y, et al. Acta Pharm Sin B. 2023 Dec;13(12):4667-4687. doi: 10.1016/j.apsb.2023.08.009. Epub 2023 Aug 14. Acta Pharm Sin B. 2023. PMID: 38045038 Free PMC article. Review. - Olfactory Three-Needle Electroacupuncture Improved Synaptic Plasticity and Gut Microbiota of SAMP8 Mice by Stimulating Olfactory Nerve.
Wang Y, Zheng AN, Yang H, Wang Q, Dai B, Wang JJ, Wan YT, Liu ZB, Liu SY. Wang Y, et al. Chin J Integr Med. 2024 Aug;30(8):729-741. doi: 10.1007/s11655-023-3614-3. Epub 2023 Nov 24. Chin J Integr Med. 2024. PMID: 37999886 - Systemic inflammatory and gut microbiota responses to fracture in young and middle-aged mice.
Roberts JL, Chiedo B, Drissi H. Roberts JL, et al. Geroscience. 2023 Dec;45(6):3115-3129. doi: 10.1007/s11357-023-00963-7. Epub 2023 Oct 12. Geroscience. 2023. PMID: 37821753 Free PMC article. - Impact of Donepezil Supplementation on Alzheimer's Disease-like Pathology and Gut Microbiome in APP/PS1 Mice.
Li Y, Wu M, Kong M, Sui S, Wang Q, He Y, Gu J. Li Y, et al. Microorganisms. 2023 Sep 13;11(9):2306. doi: 10.3390/microorganisms11092306. Microorganisms. 2023. PMID: 37764150 Free PMC article. - Spreading Senescent Cells' Burden and Emerging Therapeutic Targets for Frailty.
Marcozzi S, Bigossi G, Giuliani ME, Lai G, Giacconi R, Piacenza F, Malavolta M. Marcozzi S, et al. Cells. 2023 Sep 15;12(18):2287. doi: 10.3390/cells12182287. Cells. 2023. PMID: 37759509 Free PMC article.
References
- Alsop D. C., Detre J. A., Golay X., Günther M., Hendrikse J., Hernandez-Garcia L., et al. . (2015). Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 73, 102–116. 10.1002/mrm.25197 - DOI - PMC - PubMed
- Alvarez X. A., Mouzo R., Pichel V., Pérez P., Laredo M., Fernández-Novoa L., et al. . (1999). Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find. Exp. Clin. Pharmacol. 21, 633–644. - PubMed
- Arendash G. W., King D. L., Gordon M. N., Morgan D., Hatcher J. M., Hope C. E., et al. . (2001). Progressive, age-related behavioral impairments in transgenic mice carrying both mutant amyloid precursor protein and presenilin-1 transgenes. Brain Res. 891, 42–53. 10.1016/s0006-8993(00)03186-3 - DOI - PubMed
Grants and funding
- UL1 TR000117/TR/NCATS NIH HHS/United States
- R01 AG054459/AG/NIA NIH HHS/United States
- K01 AG040164/AG/NIA NIH HHS/United States
- R01 AG039621/AG/NIA NIH HHS/United States
- R01 NS079507/NS/NINDS NIH HHS/United States
- S10 RR029541/RR/NCRR NIH HHS/United States
- T32 DK007778/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources