The pathophysiological role of mitochondrial oxidative stress in lung diseases - PubMed (original) (raw)
Review
The pathophysiological role of mitochondrial oxidative stress in lung diseases
Xiaojing Liu et al. J Transl Med. 2017.
Abstract
Mitochondria are critically involved in reactive oxygen species (ROS)-dependent lung diseases, such as lung fibrosis, asbestos, chronic airway diseases and lung cancer. Mitochondrial DNA (mtDNA) encodes mitochondrial proteins and is more sensitive to oxidants than nuclear DNA. Damage to mtDNA causes mitochondrial dysfunction, including electron transport chain impairment and mitochondrial membrane potential loss. Furthermore, damaged mtDNA also acts as a damage-associated molecular pattern (DAMP) that drives inflammatory and immune responses. In this review, crosstalk among alveolar epithelial cells, alveolar macrophages and mitochondria is examined. ROS-related transcription factors and downstream cell signaling pathways are also discussed. We conclude that targeting oxidative stress with antioxidant agents, such as thiol molecules, polyphenols and superoxide dismutase (SOD), and promoting mitochondrial biogenesis should be considered as novel strategies for treating lung diseases that currently have no effective treatment options.
Keywords: Antioxidant agents; Mitochondrial DNA; Reactive oxygen species.
Figures
Fig. 1
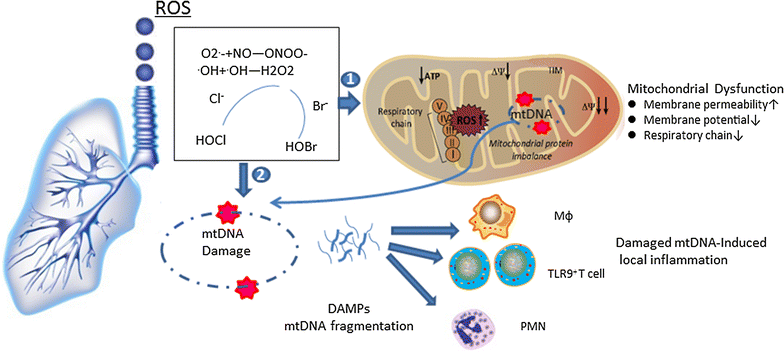
Proposed model for mitochondria-centered pathogenesis in ROS-induced lung diseases. Reactive oxygen species (ROS), commonly from exogenous exposure to smoke and air pollution, can be inhaled by the lungs. The superoxide anion radical (O2·−) can react with NO to form the highly reactive peroxynitrite molecule (ONOO−). In the presence of chloride (Cl−) and bromide (Br−) ions, the very damaging oxidants hypochlorous acid (HOCl) and hypobromous acid (HOBr) are generated from hydrogen peroxidase (H2O2). (1) ROS-induced mitochondrial dysfunction: when the concentration of ROS increases in mitochondria, the electron chain transfer (ETC) complex becomes defective and leads to mitochondrial membrane potential loss and membrane permeability increases. (2) Mitochondrial DNA (mtDNA)-associated immune response: damaged mtDNA fragments are released from mitochondria. The immunogenic particles can recruit various immune cells, such as macrophages, TLR9+-T cells and neutrophils, to the damaged area to initiate inflammatory and immunological reactions
Fig. 2
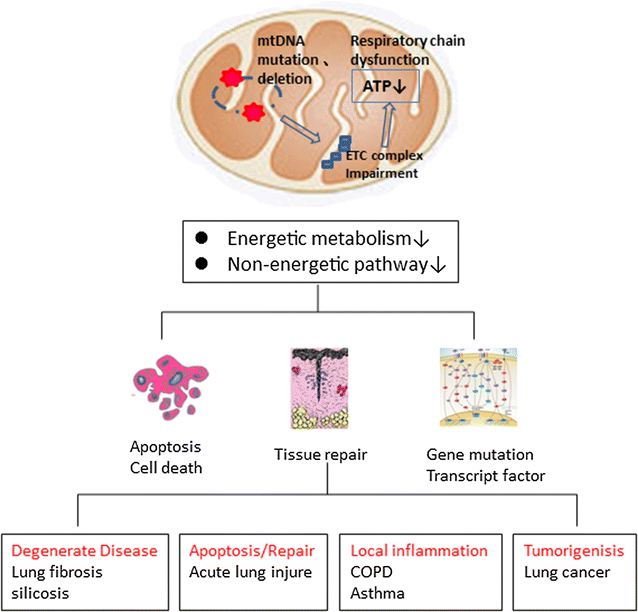
Mitochondrial dysfunction and diverse lung diseases. Mitochondrial dysfunction and mitochondrial DNA mutations and deletions can cause defects in energy metabolism and impair non-energetic pathways. Mitochondrial dysfunction is also associated with lung alveolar epithelial cell and alveolar macrophage apoptosis, which hinders lung repair and influences the expression of various reactive oxygen species-related genes. Thus, mitochondrial dysfunction can induce diverse degenerative lung diseases
Similar articles
- The Role of Mitochondrial DNA in Mediating Alveolar Epithelial Cell Apoptosis and Pulmonary Fibrosis.
Kim SJ, Cheresh P, Jablonski RP, Williams DB, Kamp DW. Kim SJ, et al. Int J Mol Sci. 2015 Sep 7;16(9):21486-519. doi: 10.3390/ijms160921486. Int J Mol Sci. 2015. PMID: 26370974 Free PMC article. Review. - [Pathways for maintenance of mitochondrial DNA integrity and mitochondrial functions in cells exposed to ionizing radiation].
Gaziev AI. Gaziev AI. Radiats Biol Radioecol. 2013 Mar-Apr;53(2):117-36. doi: 10.7868/s0869803113020045. Radiats Biol Radioecol. 2013. PMID: 23786028 Review. Russian. - Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage.
Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ. Indo HP, et al. Mitochondrion. 2007 Feb-Apr;7(1-2):106-18. doi: 10.1016/j.mito.2006.11.026. Epub 2006 Dec 13. Mitochondrion. 2007. PMID: 17307400 - Time-course effect of high-glucose-induced reactive oxygen species on mitochondrial biogenesis and function in human renal mesangial cells.
Al-Kafaji G, Sabry MA, Skrypnyk C. Al-Kafaji G, et al. Cell Biol Int. 2016 Jan;40(1):36-48. doi: 10.1002/cbin.10520. Epub 2015 Sep 1. Cell Biol Int. 2016. PMID: 26251331
Cited by
- The proteomic response is linked to regional lung volumes in ventilator-induced lung injury.
Yen S, Song Y, Preissner M, Bennett E, Wilson R, Pavez M, Dubsky S, Dargaville PA, Fouras A, Zosky GR. Yen S, et al. J Appl Physiol (1985). 2020 Oct 1;129(4):837-845. doi: 10.1152/japplphysiol.00097.2020. Epub 2020 Aug 6. J Appl Physiol (1985). 2020. PMID: 32758039 Free PMC article. - Peripheral neutrophils and oxidative stress-associated molecules for predicting the severity of asthma: a cross-sectional study based on multidimensional assessment.
Mao R, Jiang Z, Min Z, Wang G, Xie M, Gao P, Zhu L, Li H, Chen Z. Mao R, et al. Front Med (Lausanne). 2023 Dec 7;10:1240253. doi: 10.3389/fmed.2023.1240253. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38131042 Free PMC article. - Oxidant/Antioxidant Disequilibrium in Idiopathic Pulmonary Fibrosis Pathogenesis.
Cameli P, Carleo A, Bergantini L, Landi C, Prasse A, Bargagli E. Cameli P, et al. Inflammation. 2020 Feb;43(1):1-7. doi: 10.1007/s10753-019-01059-1. Inflammation. 2020. PMID: 31297749 Review. - Dark Side of Cancer Therapy: Cancer Treatment-Induced Cardiopulmonary Inflammation, Fibrosis, and Immune Modulation.
Boopathi E, Thangavel C. Boopathi E, et al. Int J Mol Sci. 2021 Sep 19;22(18):10126. doi: 10.3390/ijms221810126. Int J Mol Sci. 2021. PMID: 34576287 Free PMC article. Review. - Metabolic reprogramming of macrophages and its involvement in inflammatory diseases.
Guo C, Islam R, Zhang S, Fang J. Guo C, et al. EXCLI J. 2021 Mar 11;20:628-641. doi: 10.17179/excli2020-3053. eCollection 2021. EXCLI J. 2021. PMID: 33883988 Free PMC article. Review.
References
- Bhatia M. Understanding toxicology: mechanisms and applications. Cell Biol Toxicol. 2017;33:1–4. - PubMed
- Park HS, Kim SR, Lee YC. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. - PubMed
- Pereira AC, Martel F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol Toxicol. 2014;30:301–312. - PubMed
- Risom L, Moller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592:119–137. - PubMed
- Maynard RL. The effects on health of ambient particles: time for an agonizing reappraisal? Cell Biol Toxicol. 2015;31:131–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical