Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease - PubMed (original) (raw)
Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer's disease
Yodai Kobayashi et al. Sci Rep. 2017.
Abstract
It has previously been shown that the consumption of probiotics may have beneficial effects not only on peripheral tissues but also on the central nervous system and behavior via the microbiota-gut-brain axis, raising the possibility that treatment with probiotics could be an effective therapeutic strategy for managing neurodegenerative disorders. In this study, we investigated the effects of oral administration of Bifidobacterium breve strain A1 (B. breve A1) on behavior and physiological processes in Alzheimer's disease (AD) model mice. We found that administration of B. breve A1 to AD mice reversed the impairment of alternation behavior in a Y maze test and the reduced latency time in a passive avoidance test, indicating that it prevented cognitive dysfunction. We also demonstrated that non-viable components of the bacterium or its metabolite acetate partially ameliorated the cognitive decline observed in AD mice. Gene profiling analysis revealed that the consumption of B. breve A1 suppressed the hippocampal expressions of inflammation and immune-reactive genes that are induced by amyloid-β. Together, these findings suggest that B. breve A1 has therapeutic potential for preventing cognitive impairment in AD.
Conflict of interest statement
Authors Y.K., H.S., E.M., T.K. and J.Z.X. are the employee of Morinaga Milk Industry Co., Ltd.
Figures
Figure 1
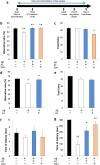
Effect of Bifidobacterium breve strain A1 treatment on cognitive function in AD model mice evaluated by Y maze test and passive avoidance test. (a) Experimental design of the mouse study. An animal model of AD was induced by intracerebroventricular (ICV) injection of Aβ25–35 or Aβ1–42. The probiotics was orally administered every day starting 2 days before ICV injection. 6 days after ICV, cognitive function was evaluated by Y maze test, thereafter the mice received passive avoidance test. (b) Alternative ratio in Y maze test. (c) Total entry time in Y maze test. (d) Alternative ratio and (e) Total entry time in Y maze test of Aβ1–42 injected mice. (f) The latency time of acquisition trial. (g) The latency time of testing session. For (b,c,f,g), mice were injected Aβ25–35. n = 10 mice in each group. For (d,e), Aβ1–42 was ICV injected, n = 11–12 mice in each group. †P < 0.05, ††P < 0.01 vs. control (sham). *P < 0.05, **P < 0.01 vs. Aβ ( + ). All values are expressed as mean ± S.E.. A1: B. breve A1, Don: Donepezil.
Figure 2
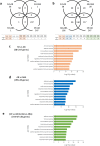
Change of gene expression profile in hippocampus of AD model mice by Bifidobacterium breve strain A1 treatment using RNA-seq analysis. Transcriptional analysis was performed on hippocampal tissues of sham-operated mice (SH), Aβ25-35 injected mice (AB) and mice treated with Aβ and B breve A1 (ABA). (a,b) Venn diagram of shared and unique hippocampal transcripts (a) in SH vs AB and/or SH vs ABA, and (b) in SH vs AB and/or AB vs ABA, p < 0.05 and FDR < 0.05. RNA-seq data from 5 mice are presented. (c–e) GO Term enrichment analysis of differential expressed (DE) genes in AD hippocampus. Enrichment analysis of differential expressed genes (c) between Aβ-treated and control mice, (d) between groups with or without B. breve A1 administration and (e) using DAVID analysis.
Figure 3
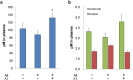
Plasma SCFA levels of AD model mice. (a,b) Plasma SCFA levels of AD model mice for acetate (a) and for propionate and butylate (b). N = 4 for each group. *P < 0.05 vs. Aβ ( + ). All values are expressed as mean ± S.E.. A1: B. breve A1.
Figure 4
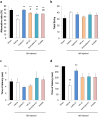
Effect of acetate treatment and non-viable Bifidobacterium breve A1 treatment on cognitive function in AD model mice. (a) Alternative ratio in Y maze test. (b) Total entry time in Y maze test. (c) The latency time of acquisition trial. (d) The latency time of testing session. Mice were injected Aβ25-35. n = 10 mice in each group. ††P < 0.01 vs. control. **P < 0.01 vs. Aβ ( + ). #P < 0.05, ##P < 0.01 vs. viable B. breve A1 group. All values are expressed as mean ± S.E.
Similar articles
- Bifidobacterium Breve A1 Supplementation Improved Cognitive Decline in Older Adults with Mild Cognitive Impairment: An Open-Label, Single-Arm Study.
Kobayashi Y, Kinoshita T, Matsumoto A, Yoshino K, Saito I, Xiao JZ. Kobayashi Y, et al. J Prev Alzheimers Dis. 2019;6(1):70-75. doi: 10.14283/jpad.2018.32. J Prev Alzheimers Dis. 2019. PMID: 30569089 Clinical Trial. - Bifidobacterium breve HNXY26M4 Attenuates Cognitive Deficits and Neuroinflammation by Regulating the Gut-Brain Axis in APP/PS1 Mice.
Zhu G, Zhao J, Wang G, Chen W. Zhu G, et al. J Agric Food Chem. 2023 Mar 22;71(11):4646-4655. doi: 10.1021/acs.jafc.3c00652. Epub 2023 Mar 8. J Agric Food Chem. 2023. PMID: 36888896 - Protective Effects of Bifidobacterium Breve MCC1274 as a Novel Therapy for Alzheimer's Disease.
Abdelhamid M, Counts SE, Zhou C, Hida H, Kim JI, Michikawa M, Jung CG. Abdelhamid M, et al. Nutrients. 2025 Jan 31;17(3):558. doi: 10.3390/nu17030558. Nutrients. 2025. PMID: 39940416 Free PMC article. Review. - Bifidobacterium breve intervention combined with environmental enrichment alleviates cognitive impairment by regulating the gut microbiota and microbial metabolites in Alzheimer's disease mice.
Zhu G, Guo M, Zhao J, Zhang H, Wang G, Chen W. Zhu G, et al. Front Immunol. 2022 Sep 20;13:1013664. doi: 10.3389/fimmu.2022.1013664. eCollection 2022. Front Immunol. 2022. PMID: 36203603 Free PMC article. - Bifidobacterium infantis and Bifidobacterium breve Improve Symptomatology and Neuronal Damage in Neurodegenerative Disease: A Systematic Review.
Reiriz M, Beltrán-Velasco AI, Echeverry-Alzate V, Martínez-Miguel E, Gómez-Senent S, Uceda S, Clemente-Suárez VJ. Reiriz M, et al. Nutrients. 2025 Jan 22;17(3):391. doi: 10.3390/nu17030391. Nutrients. 2025. PMID: 39940249 Free PMC article.
Cited by
- Immunoregulatory Effects of Tolerogenic Probiotics in Multiple Sclerosis.
Atabati H, Yazdanpanah E, Mortazavi H, Bajestani SG, Raoofi A, Esmaeili SA, Khaledi A, Saburi E, Afshari JT, Sathyapalan T, Moghaddam AS, Sahebkar A. Atabati H, et al. Adv Exp Med Biol. 2021;1286:87-105. doi: 10.1007/978-3-030-55035-6_6. Adv Exp Med Biol. 2021. PMID: 33725347 Review. - Gut Microbiota Interaction with the Central Nervous System throughout Life.
Ojeda J, Ávila A, Vidal PM. Ojeda J, et al. J Clin Med. 2021 Mar 21;10(6):1299. doi: 10.3390/jcm10061299. J Clin Med. 2021. PMID: 33801153 Free PMC article. Review. - Effects of probiotic supplements on cognition, anxiety, and physical activity in subjects with mild and moderate Alzheimer's disease: A randomized, double-blind, and placebo-controlled study.
Akhgarjand C, Vahabi Z, Shab-Bidar S, Etesam F, Djafarian K. Akhgarjand C, et al. Front Aging Neurosci. 2022 Oct 31;14:1032494. doi: 10.3389/fnagi.2022.1032494. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36389063 Free PMC article. - Non-Pharmacological Therapeutic Options for the Treatment of Alzheimer's Disease.
Huynh QS, Elangovan S, Holsinger RMD. Huynh QS, et al. Int J Mol Sci. 2022 Sep 20;23(19):11037. doi: 10.3390/ijms231911037. Int J Mol Sci. 2022. PMID: 36232336 Free PMC article. Review. - Microbial composition, functionality, and stress resilience or susceptibility: unraveling sex-specific patterns.
Tanelian A, Nankova B, Miari M, Sabban EL. Tanelian A, et al. Biol Sex Differ. 2024 Feb 26;15(1):20. doi: 10.1186/s13293-024-00590-7. Biol Sex Differ. 2024. PMID: 38409102 Free PMC article.
References
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat. med. 2006;12:1005–1015. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical