An open-label pilot study of an intervention using mobile phones to deliver contingency management of tobacco abstinence to high school students - PubMed (original) (raw)
An open-label pilot study of an intervention using mobile phones to deliver contingency management of tobacco abstinence to high school students
Grace Kong et al. Exp Clin Psychopharmacol. 2017 Oct.
Abstract
This pilot study assessed the feasibility, acceptability, and preliminary efficacy of a smoking cessation intervention that used mobile phones to remotely deliver reinforcements contingent on tobacco abstinence (contingency management [CM]) and weekly in-person cognitive-behavioral therapy to adolescent smokers. Daily adolescent smokers (N = 15; 12 completed study procedures, 3 dropped out) were recruited to participate in a 4-week study. During the first 2 weeks, daily text messages sent at random times prompted participants to transmit a video of themselves providing a carbon monoxide (CO) sample. During the last 2 weeks, text messages sent on 3 randomly chosen days each week prompted participants to transmit a video of themselves providing a saliva sample. Negative samples (CO ≤8 ppm; cotinine ≤100 ng/ml) were reinforced with monetary incentives. Feasibility was assessed using the number of on-time, valid videos, and acceptability was determined using participant perceptions of the intervention. Seven-day point-prevalence (PP) abstinence (self-reported abstinence, cotinine <100 ng/ml) was assessed at end of treatment (EOT) and at a 1-month follow-up. The study findings indicate that remote delivery of CM using mobile phones was feasible (85.8% of the CO videos and 67% of the saliva cotinine videos were on time) and acceptable (positive perceptions of the CM procedures). Seven-day PP was 60% at EOT and 46.7% at the follow-up. Although larger randomized controlled trials are needed to evaluate efficacy, this pilot study suggests that the use of mobile phones to deliver CM for smoking cessation among adolescent smokers was acceptable and feasible. (PsycINFO Database Record
(c) 2017 APA, all rights reserved).
Figures
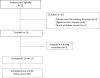
Figure 1. Reasons for exclusion and flow of participants from initial contact to follow up
*One participant who dropped out during treatment completed the follow up assessment.
Similar articles
- An exploratory randomized controlled trial of a novel high-school-based smoking cessation intervention for adolescent smokers using abstinence-contingent incentives and cognitive behavioral therapy.
Krishnan-Sarin S, Cavallo DA, Cooney JL, Schepis TS, Kong G, Liss TB, Liss AK, McMahon TJ, Nich C, Babuscio T, Rounsaville BJ, Carroll KM. Krishnan-Sarin S, et al. Drug Alcohol Depend. 2013 Sep 1;132(1-2):346-51. doi: 10.1016/j.drugalcdep.2013.03.002. Epub 2013 Mar 22. Drug Alcohol Depend. 2013. PMID: 23523130 Free PMC article. Clinical Trial. - The efficacy of mobile phone-based text message interventions ('Happy Quit') for smoking cessation in China.
Liao Y, Wu Q, Tang J, Zhang F, Wang X, Qi C, He H, Long J, Kelly BC, Cohen J. Liao Y, et al. BMC Public Health. 2016 Aug 19;16(1):833. doi: 10.1186/s12889-016-3528-5. BMC Public Health. 2016. PMID: 27543164 Free PMC article. Clinical Trial. - Financial incentives to promote extended smoking abstinence in opioid-maintained patients: a randomized trial.
Sigmon SC, Miller ME, Meyer AC, Saulsgiver K, Badger GJ, Heil SH, Higgins ST. Sigmon SC, et al. Addiction. 2016 May;111(5):903-12. doi: 10.1111/add.13264. Epub 2016 Jan 23. Addiction. 2016. PMID: 26638126 Free PMC article. Clinical Trial. - A Scoping Review and Meta-analysis of the Use of Remote Biochemical Verification Methods of Smoking Status in Tobacco Research.
Thrul J, Howe CL, Devkota J, Alexander A, Allen AM, Businelle MS, Hébert ET, Heffner JL, Kendzor DE, Ra CK, Gordon JS. Thrul J, et al. Nicotine Tob Res. 2023 Jul 14;25(8):1413-1423. doi: 10.1093/ntr/ntac271. Nicotine Tob Res. 2023. PMID: 36449414 Free PMC article. - Recent marijuana blunt smoking impacts carbon monoxide as a measure of adolescent tobacco abstinence.
Moolchan ET, Zimmerman D, Sehnert SS, Zimmerman D, Huestis MA, Epstein DH. Moolchan ET, et al. Subst Use Misuse. 2005;40(2):231-40. doi: 10.1081/ja-200048461. Subst Use Misuse. 2005. PMID: 15770886 Review.
Cited by
- Review of strategies to investigate low sample return rates in remote tobacco trials: A call to action for more user-centered design research.
Vilardaga R, Thrul J, DeVito A, Kendzor DE, Sabo P, Khafif TC. Vilardaga R, et al. Addict Neurosci. 2023 Sep;7:100090. doi: 10.1016/j.addicn.2023.100090. Epub 2023 Apr 11. Addict Neurosci. 2023. PMID: 37424632 Free PMC article. - Interest in and perceived effectiveness of contingency management among alcohol drinkers using behavioral economic purchase tasks.
Traxler HK, Kaplan BA, Rzeszutek MJ, Franck CT, Koffarnus MN. Traxler HK, et al. Exp Clin Psychopharmacol. 2023 Feb;31(1):127-139. doi: 10.1037/pha0000580. Epub 2022 Jun 16. Exp Clin Psychopharmacol. 2023. PMID: 35708948 Free PMC article. - Automated Mobile Delivery of Financial Incentives for Smoking Cessation Among Socioeconomically Disadvantaged Adults: Feasibility Study.
Kendzor DE, Businelle MS, Waring JJC, Mathews AJ, Geller DW, Barton JM, Alexander AC, Hébert ET, Ra CK, Vidrine DJ. Kendzor DE, et al. JMIR Mhealth Uhealth. 2020 Apr 15;8(4):e15960. doi: 10.2196/15960. JMIR Mhealth Uhealth. 2020. PMID: 32293568 Free PMC article. - Establishing a Working Definition of User Experience for eHealth Interventions of Self-reported User Experience Measures With eHealth Researchers and Adolescents: Scoping Review.
Newton AS, March S, Gehring ND, Rowe AK, Radomski AD. Newton AS, et al. J Med Internet Res. 2021 Dec 2;23(12):e25012. doi: 10.2196/25012. J Med Internet Res. 2021. PMID: 34860671 Free PMC article. - Mobile contingency management for smoking cessation among socioeconomically disadvantaged adults: Protocol for a randomized trial.
Kendzor DE, Businelle MS, Vidrine DJ, Frank-Pearce SG, Shih YT, Dallery J, Alexander AC, Boozary LK, Waring JJC, Ehlke SJ. Kendzor DE, et al. Contemp Clin Trials. 2022 Mar;114:106701. doi: 10.1016/j.cct.2022.106701. Epub 2022 Jan 31. Contemp Clin Trials. 2022. PMID: 35114409 Free PMC article.
References
- Backinger CL, Leischow SJ. Advancing the science of adolescent tobacco use cessation. American Journal of Health Behavior. 2001;25(3):183–190. - PubMed
- Cavallo DA, Cooney JL, Duhig AM, Smith AE, Liss TB, McFetridge AK, Krishnan-Sarin S. Combining cognitive behavioral therapy with contingency management for smoking cessation in adolescent smokers: a preliminary comparison of two different CBT formats. American Journal on Addictions. 2007;16(6):468–474. doi: 10.1080/10550490701641173. - DOI - PMC - PubMed
- Dallery J, Glenn IM, Raiff BR. An Internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence. 2007;86:230–238. http://dx.doi.org/10.1016/j.drugalcdep.2006.06.013. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- P30 DA029926/DA/NIDA NIH HHS/United States
- UL1 TR000142/TR/NCATS NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- National Institutes of Health; National Center for Advancing Translational Science; NIH roadmap for Medical Research/International
- KL2 TR000140/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous