FL-926-16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice - PubMed (original) (raw)
FL-926-16, a novel bioavailable carnosinase-resistant carnosine derivative, prevents onset and stops progression of diabetic nephropathy in db/db mice
Carla Iacobini et al. Br J Pharmacol. 2018 Jan.
Abstract
Background and purpose: The advanced glycation end products (AGEs) participate in the pathogenesis of diabetic nephropathy (DN) by promoting renal inflammation and injury. L-carnosine acts as a quencher of the AGE precursors reactive carbonyl species (RCS), but is rapidly inactivated by carnosinase. In this study, we evaluated the effect of FL-926-16, a carnosinase-resistant and bioavailable carnosine derivative, on the onset and progression of DN in db/db mice.
Experimental approach: Adult male db/db mice and coeval db/m controls were left untreated or treated with FL-926-16 (30 mg·kg-1 body weight) from weeks 6 to 20 (prevention protocol) or from weeks 20 to 34 (regression protocol).
Key results: In the prevention protocol, FL-926-16 significantly attenuated increases in creatinine (-80%), albuminuria (-77%), proteinuria (-75%), mean glomerular area (-34%), fractional (-40%) and mean (-42%) mesangial area in db/db mice. This protective effect was associated with a reduction in glomerular matrix protein expression and cell apoptosis, circulating and tissue oxidative and carbonyl stress, and renal inflammatory markers, including the NLRP3 inflammasome. In the regression protocol, the progression of DN was completely blocked, although not reversed, by FL-926-16. In cultured mesangial cells, FL-926-16 prevented NLRP3 expression induced by RCS but not by the AGE Nε -carboxymethyllysine.
Conclusion and implications: FL-926-16 is effective at preventing the onset of DN and halting its progression in db/db mice by quenching RCS, thereby reducing the accumulation of their protein adducts and the consequent inflammatory response. In a future perspective, this novel compound may represent a promising AGE-reducing approach for DN therapy.
© 2017 The British Pharmacological Society.
Figures
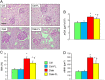
Figure 1
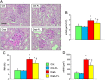
Prevention protocol: renal structure. PAS staining of kidney sections from representative animals (A) and quantification of mGA (B), fMA (C) and mMA (D) in untreated and FL‐926‐16 (FL)‐treated db/m control (Ctrl) and db/db diabetic (Diab) mice (mean ± SD; n = 10 per group). Scale bar = 50 μm. Post hoc multiple comparison: *P < 0.05 versus Ctrl; †P < 0.05 versus Diab.
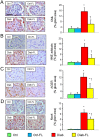
Figure 2
Prevention protocol: glomerular extracellular matrix and cell apoptosis. IHC of kidney sections from representative animals and quantification of fibronectin (A) and collagen IV α1 chain (B); kidney mRNA expression of FN1 (C), Col4a1 (D) and Tgfb (E); IHC of kidney sections (original quantification 1000×) from representative animals and quantification of active caspase‐3 (F, arrows indicate positive cells) in untreated and FL‐926‐16 (FL)‐treated db/m control (Ctrl) and db/db diabetic (Diab) mice (mean ± SD; n = 5 per group). Scale bar = 50 μm. Post hoc multiple comparison: *P < 0.05 versus Ctrl; †P < 0.05 versus Diab.
Figure 3
Prevention protocol: renal oxidative and carbonyl stress. IHC of kidney sections from representative animals and quantification of CML (A), HNE (B), AGER (C) and Nox4 (D) in untreated and FL‐926‐16 (FL)‐treated db/m control (Ctrl) and db/db diabetic (Diab) mice (mean ± SD; n = 5 per group). Scale bar = 50 μm. Post hoc multiple comparison: *P < 0.05 versus Ctrl; †P < 0.05 versus Diab.
Figure 4
Prevention protocol: renal inflammation. IHC of kidney sections from representative animals and quantification of F4/80 (A) and MCP‐1 (B) and kidney mRNA expression of Adgre1 (C), Mcp1 (D) and Tnfa (E) in untreated and FL‐926‐16 (FL)‐treated db/m control (Ctrl) and db/db diabetic (Diab) mice (mean ± SD; n = 5 per group). Scale bar = 50 μm. Post hoc multiple comparison: *P < 0.05 versus Ctrl, †P < 0.05 versus Diab.
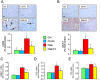
Figure 5
In vivo and in vitro studies: NLRP3 expression. IHC of kidney sections from representative animals and quantification of NLRP3 (A) and kidney mRNA expression of Nlrp3 (B) in untreated and FL‐926‐16 (FL)‐treated db/m control (Ctrl) and db/db diabetic (Diab) mice (mean ± SD; n = 5 per group). Scale bar = 50 μm. Post hoc multiple comparison: *P < 0.05 versus Ctrl; †P < 0.001 versus Diab. Expression levels of Nlrp3 in mouse mesangial cells incubated with vehicle (Ctrl), 100 μg·mL−1 CML, 100 μM glyoxal (Gly) or 5 μM HNE, without and with FL‐926‐16 20 mM (C) (mean ± SD; n = 5 experiments in triplicate). Pairwise comparison: *P < 0.05 versus Ctrl; †P < 0.05 versus untreated.
Figure 6
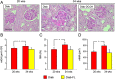
Regression protocol: renal structure. PAS staining of kidney sections from representative animals (A) and quantification of mGA (B), fMA (C) and mMA (D) in untreated and FL‐926‐16 (FL)‐treated db/m control (Ctrl) and db/db diabetic (Diab) mice (mean ± SD; n = 5 per group). Scale bar = 50 μm. Post hoc multiple comparison: *P < 0.05 versus Ctrl, †P < 0.05 versus Diab.
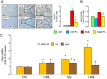
Figure 7
Prevention and regression protocols: renal structure. PAS staining of kidney sections from representative animals (A) and quantification of mGA (B), fMA (C) and mMA (D) in untreated and FL‐926‐16 (FL)‐treated db/db diabetic mice (Diab and Diab‐FL) of 34 weeks of age versus untreated Diab mice of 20 weeks of age (mean ± SD; n = 10 for 20‐week‐old mice and 5 for 34‐week‐old mice). Scale bar = 50 μm. Pairwise comparison: *P < 0.05 versus Diab‐20 weeks.
Similar articles
- D-Carnosine octylester attenuates atherosclerosis and renal disease in ApoE null mice fed a Western diet through reduction of carbonyl stress and inflammation.
Menini S, Iacobini C, Ricci C, Scipioni A, Blasetti Fantauzzi C, Giaccari A, Salomone E, Canevotti R, Lapolla A, Orioli M, Aldini G, Pugliese G. Menini S, et al. Br J Pharmacol. 2012 Jun;166(4):1344-56. doi: 10.1111/j.1476-5381.2012.01834.x. Br J Pharmacol. 2012. PMID: 22229552 Free PMC article. - Carnosine treatment largely prevents alterations of renal carnosine metabolism in diabetic mice.
Peters V, Schmitt CP, Zschocke J, Gross ML, Brismar K, Forsberg E. Peters V, et al. Amino Acids. 2012 Jun;42(6):2411-6. doi: 10.1007/s00726-011-1046-4. Epub 2011 Aug 11. Amino Acids. 2012. PMID: 21833769 - Carnosine alleviates diabetic nephropathy by targeting GNMT, a key enzyme mediating renal inflammation and fibrosis.
Liu XQ, Jiang L, Lei L, Nie ZY, Zhu W, Wang S, Zeng HX, Zhang SQ, Zhang Q, Yard B, Wu YG. Liu XQ, et al. Clin Sci (Lond). 2020 Dec 11;134(23):3175-3193. doi: 10.1042/CS20201207. Clin Sci (Lond). 2020. PMID: 33241846 Free PMC article. - Carnosine and Diabetic Nephropathy.
Peters V, Yard B, Schmitt CP. Peters V, et al. Curr Med Chem. 2020;27(11):1801-1812. doi: 10.2174/0929867326666190326111851. Curr Med Chem. 2020. PMID: 30914013 Review. - L-carnosine and its Derivatives as New Therapeutic Agents for the Prevention and Treatment of Vascular Complications of Diabetes.
Menini S, Iacobini C, Fantauzzi CB, Pugliese G. Menini S, et al. Curr Med Chem. 2020;27(11):1744-1763. doi: 10.2174/0929867326666190711102718. Curr Med Chem. 2020. PMID: 31296153 Review.
Cited by
- PEGylation renders carnosine resistant to hydrolysis by serum carnosinase and increases renal carnosine levels.
Zhang S, Yang G, Zhang Q, Fan Y, Tang M, Shen L, Zhu D, Zhang G, Yard B. Zhang S, et al. Amino Acids. 2024 Jul 4;56(1):44. doi: 10.1007/s00726-024-03405-6. Amino Acids. 2024. PMID: 38960916 Free PMC article. - Roles of Inflammasomes in Inflammatory Kidney Diseases.
Fan J, Xie K, Wang L, Zheng N, Yu X. Fan J, et al. Mediators Inflamm. 2019 Jul 21;2019:2923072. doi: 10.1155/2019/2923072. eCollection 2019. Mediators Inflamm. 2019. PMID: 31427885 Free PMC article. Review. - Protective Actions of Anserine Under Diabetic Conditions.
Peters V, Calabrese V, Forsberg E, Volk N, Fleming T, Baelde H, Weigand T, Thiel C, Trovato A, Scuto M, Modafferi S, Schmitt CP. Peters V, et al. Int J Mol Sci. 2018 Sep 13;19(9):2751. doi: 10.3390/ijms19092751. Int J Mol Sci. 2018. PMID: 30217069 Free PMC article. - 3D autofluorescence imaging of hydronephrosis and renal anatomical structure using cryo-micro-optical sectioning tomography.
Fan G, Jiang C, Huang Z, Tian M, Pan H, Cao Y, Lei T, Luo Q, Yuan J. Fan G, et al. Theranostics. 2023 Sep 4;13(14):4885-4904. doi: 10.7150/thno.86695. eCollection 2023. Theranostics. 2023. PMID: 37771780 Free PMC article. - Carnosinase-1 Knock-Out Reduces Kidney Fibrosis in Type-1 Diabetic Mice on High Fat Diet.
Pfeffer T, Wetzel C, Kirschner P, Bartosova M, Poth T, Schwab C, Poschet G, Zemva J, Bulkescher R, Damgov I, Thiel C, Garbade SF, Klingbeil K, Peters V, Schmitt CP. Pfeffer T, et al. Antioxidants (Basel). 2023 Jun 14;12(6):1270. doi: 10.3390/antiox12061270. Antioxidants (Basel). 2023. PMID: 37372000 Free PMC article.
References
- Ahluwalia TS, Lindholm E, Groop LC (2011). Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes. Diabetologia 54: 2295–2302. - PubMed
- Ahmed N (2005). Advanced glycation endproducts – role in pathology of diabetic complications. Diabetes Res Clin Pract 67: 3–21. - PubMed
- Aldini G, Facino RM, Beretta G, Carini M (2005). Carnosine and related dipeptides as quenchers of reactive carbonyl species: from structural studies to therapeutic perspectives. Biofactors 24: 77–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous