N-acetyl-L-cysteine functionalized nanostructured lipid carrier for improving oral bioavailability of curcumin: preparation, in vitro and in vivo evaluations - PubMed (original) (raw)
N-acetyl-L-cysteine functionalized nanostructured lipid carrier for improving oral bioavailability of curcumin: preparation, in vitro and in vivo evaluations
Cihui Tian et al. Drug Deliv. 2017 Nov.
Abstract
The application of orally administered nanoparticles in the circulation system is limited by the secretion and shedding of intestinal tract mucous layer. In order to enhance mucoadhesion and mucus penetration of curcumin (Cur)-loaded nanostructured lipid carrier (NLC) after oral administration, a new multifunctional conjugate, N-acetyl-L-cysteine-polyethylene glycol (100)-monostearate (NAPG), was synthesized. Functionalized nanocarriers (Cur-NAPG-NLC) modified by different amounts of NAPG (the amounts of NAPG were 20, 50, and 100 mg) were prepared and investigated for in vitro and in vivo behavior. Mean particle sizes of 89-141 nm with negative zeta potential (-15 to -11 mV) and high encapsulation efficiency (EE, >90%) possessing spherical and stable nanocarriers were observed. Sustained drug release was also observed for the NAPG-NLC. In situ intestinal perfusion studies showed that with increasing the amount of NAPG increase absorption of Cur. In vivo oral pharmacokinetic evaluation suggested that the bioavailability of Cur in rats was proportional to the degree of functionalization of NLCs with NAPG. AUC0-t of Cur-NAPG100-NLC was improved by 499.45 and 116.89 folds as compared to that of Cur solution and unmodified Cur-NLC, respectively. In conclusion, NAPG modified NLC could be a promising drug delivery system for improving oral performance of BCS class IV drugs.
Keywords: N-acetyl-L-cysteine-polyethylene glycol (100)-monostearate; curcumin; intestinal mucus layer; nanostructure lipid carrier; oral delivery.
Conflict of interest statement
The authors report no declarations of interest.
Figures
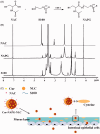
Figure 1.
(A) Synthesis of NAPG; (B) 1H-NMR spectrum of NAC, S100, and NAPG in D2O at 25 °C; (C) schematic illustration of Cur-NAPG-NLC for oral delivery of Cur by the formation of disulfide bond between NAPG with cysteine in mucus layer and PEG coating.
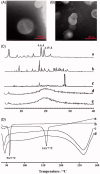
Figure 2.
The TEM images of (A ) Cur-NLC and (B) Cur-NAPG50-NLC; (C) Power X-ray diffraction patterns of: Cholesterol oleate (a), Cur (b), physical mixture of Cur and NAPG50-NLC (c), blank NAPG50-NLC (d) and Cur-NAPG50-NLC (e); (D) DSC curves of: Cur(a), NAPG (b), blank-NAPG50-NLC (c) and Cur-NAPG50-NLC (d).
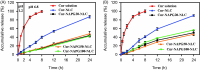
Figure 3.
In vitro cumulative drug release profiles from Cur solution, Cur-loaded NLC and NAPG-NLC in (A) pH 1.2 hydrochloric acid solution for 2 h and in pH 6.8 PBS for next 22 h and (B) physiological saline containing 1% tween 80 at 37 °C (mean ± SD; n = 3).
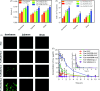
Figure 4.
In situ single-pass perfusion, (A) Ka and (B) Peff of duodenum, jejunum and ileum. *p < .05 versus Cur solution, #p < .05 versus Cur-NLC (mean ± SD; n = 3). (C) Distribution of C6 labeled different NLC in duodenum, jejunum, and ileum after oral gavage to mice by CLSM. (D) Plasma concentration–time profiles of Cur in rats after gavage administration of Cur solution, Cur-NLC, and different Cur-NAPG-NLC at a dose of 50 mg/kg of Cur, and intravenous injection of Cur solution at a dose of 2 mg/kg of Cur. Data were presented as the mean ± SD (n = 6).
Similar articles
- Improving intestinal absorption and oral bioavailability of curcumin via taurocholic acid-modified nanostructured lipid carriers.
Tian C, Asghar S, Wu Y, Chen Z, Jin X, Yin L, Huang L, Ping Q, Xiao Y. Tian C, et al. Int J Nanomedicine. 2017 Oct 27;12:7897-7911. doi: 10.2147/IJN.S145988. eCollection 2017. Int J Nanomedicine. 2017. PMID: 29138557 Free PMC article. - Curcumin-loaded solid lipid nanoparticles with Brij78 and TPGS improved in vivo oral bioavailability and in situ intestinal absorption of curcumin.
Ji H, Tang J, Li M, Ren J, Zheng N, Wu L. Ji H, et al. Drug Deliv. 2016;23(2):459-70. doi: 10.3109/10717544.2014.918677. Epub 2014 Jun 3. Drug Deliv. 2016. PMID: 24892628 - Potential advantages of a novel chitosan-N-acetylcysteine surface modified nanostructured lipid carrier on the performance of ophthalmic delivery of curcumin.
Liu D, Li J, Pan H, He F, Liu Z, Wu Q, Bai C, Yu S, Yang X. Liu D, et al. Sci Rep. 2016 Jun 28;6:28796. doi: 10.1038/srep28796. Sci Rep. 2016. PMID: 27350323 Free PMC article. - In vivo Antiplasmodial Activity of Curcumin-Loaded Nanostructured Lipid Carriers.
Rashidzadeh H, Salimi M, Sadighian S, Rostamizadeh K, Ramazani A. Rashidzadeh H, et al. Curr Drug Deliv. 2019;16(10):923-930. doi: 10.2174/1567201816666191029121036. Curr Drug Deliv. 2019. PMID: 31663477
Cited by
- Role of nanostructures in improvising oral medicine.
Ghosh S, Ghosh S, Sil PC. Ghosh S, et al. Toxicol Rep. 2019 Apr 15;6:358-368. doi: 10.1016/j.toxrep.2019.04.004. eCollection 2019. Toxicol Rep. 2019. PMID: 31080743 Free PMC article. Review. - Leveraging nanostructured lipid carriers to enhance targeted delivery and efficacy in breast cancer therapy: a comprehensive review.
Naseem N, Kushwaha P, Haider F. Naseem N, et al. Naunyn Schmiedebergs Arch Pharmacol. 2024 Aug 28. doi: 10.1007/s00210-024-03408-w. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 39196394 Review. - Antiviral effect of phytochemicals from medicinal plants: Applications and drug delivery strategies.
Ben-Shabat S, Yarmolinsky L, Porat D, Dahan A. Ben-Shabat S, et al. Drug Deliv Transl Res. 2020 Apr;10(2):354-367. doi: 10.1007/s13346-019-00691-6. Drug Deliv Transl Res. 2020. PMID: 31788762 Free PMC article. Review. - Lipid-engineered nanotherapeutics for cancer management.
Fernandez-Fernandez A, Manchanda R, Kumari M. Fernandez-Fernandez A, et al. Front Pharmacol. 2023 Mar 23;14:1125093. doi: 10.3389/fphar.2023.1125093. eCollection 2023. Front Pharmacol. 2023. PMID: 37033603 Free PMC article. Review. - Thiolated Nanoparticles for Biomedical Applications: Mimicking the Workhorses of Our Body.
Hock N, Racaniello GF, Aspinall S, Denora N, Khutoryanskiy VV, Bernkop-Schnürch A. Hock N, et al. Adv Sci (Weinh). 2022 Jan;9(1):e2102451. doi: 10.1002/advs.202102451. Epub 2021 Nov 12. Adv Sci (Weinh). 2022. PMID: 34773391 Free PMC article. Review.
References
- Anand P, Kunnumakkara AB, Newman RA, et al. . (2007). Bioavailability of curcumin: problems and promises. Mol Pharm 4:807–18. - PubMed
- Balimane PV, Chong S, Morrison RA. (2000). Current methodologies used for evaluation of intestinal permeability and absorption. J Pharmacol Toxicol Methods 44:301–12. - PubMed
- Beloqui A, Solinís MÁ, Rodríguez-Gascón A, et al. . (2016). Nanostructured lipid carriers: promising drug delivery systems for future clinics. Nanomed Nanotechnol 12:143–61. - PubMed
- Berginc K, Trontelj J, Basnet NS, et al. . (2012). Physiological barriers to the oral delivery of curcumin. Pharmazie 67:518–24. - PubMed
- Bernkop-Schnürch A, Guggi D, Pinter Y. (2004a). Thiolated chitosans: development and in vitro evaluation of a mucoadhesive, permeation enhancing oral drug delivery system. J Control Release 94:177–86. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources