Survival and divergence in a small group: The extraordinary genomic history of the endangered Apennine brown bear stragglers - PubMed (original) (raw)
. 2017 Nov 7;114(45):E9589-E9597.
doi: 10.1073/pnas.1707279114. Epub 2017 Oct 24.
Emiliano Trucchi 1 2, James A Cahill 3, Pierpaolo Maisano Delser 4 5 6, Stefano Mona 4 5, Matteo Fumagalli 7, Lynsey Bunnefeld 8 9, Luca Cornetti 10, Silvia Ghirotto 1, Matteo Girardi 11, Lino Ometto 12 13, Alex Panziera 1, Omar Rota-Stabelli 12, Enrico Zanetti 1, Alexandros Karamanlidis 14, Claudio Groff 15, Ladislav Paule 16, Leonardo Gentile 17, Carles Vilà 18, Saverio Vicario 19, Luigi Boitani [ 20](#full-view-affiliation-20 "Department of Biology and Biotechnologies "Charles Darwin," University of Rome La Sapienza, 00185 Rome, Italy."), Ludovic Orlando 21, Silvia Fuselli 1, Cristiano Vernesi 11, Beth Shapiro 3, Paolo Ciucci [ 20](#full-view-affiliation-20 "Department of Biology and Biotechnologies "Charles Darwin," University of Rome La Sapienza, 00185 Rome, Italy."), Giorgio Bertorelle 22
Affiliations
- PMID: 29078308
- PMCID: PMC5692547
- DOI: 10.1073/pnas.1707279114
Survival and divergence in a small group: The extraordinary genomic history of the endangered Apennine brown bear stragglers
Andrea Benazzo et al. Proc Natl Acad Sci U S A. 2017.
Abstract
About 100 km east of Rome, in the central Apennine Mountains, a critically endangered population of ∼50 brown bears live in complete isolation. Mating outside this population is prevented by several 100 km of bear-free territories. We exploited this natural experiment to better understand the gene and genomic consequences of surviving at extremely small population size. We found that brown bear populations in Europe lost connectivity since Neolithic times, when farming communities expanded and forest burning was used for land clearance. In central Italy, this resulted in a 40-fold population decline. The overall genomic impact of this decline included the complete loss of variation in the mitochondrial genome and along long stretches of the nuclear genome. Several private and deleterious amino acid changes were fixed by random drift; predicted effects include energy deficit, muscle weakness, anomalies in cranial and skeletal development, and reduced aggressiveness. Despite this extreme loss of diversity, Apennine bear genomes show nonrandom peaks of high variation, possibly maintained by balancing selection, at genomic regions significantly enriched for genes associated with immune and olfactory systems. Challenging the paradigm of increased extinction risk in small populations, we suggest that random fixation of deleterious alleles (i) can be an important driver of divergence in isolation, (ii) can be tolerated when balancing selection prevents random loss of variation at important genes, and (iii) is followed by or results directly in favorable behavioral changes.
Keywords: Neolithic impact; Ursus arctos; balancing selection; genetic drift; genetic load.
Published under the PNAS license.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
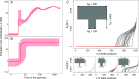
Phylogenetic relationships and patterns of homozygosity of the European brown bear. (A) Geographic distribution of the brown bear samples sequenced in this study: SLK (brown), ALP (light brown), SPA (yellow), APN (red), and Greece (GRE; green). Samples from previous studies: polar bear from Greenland (POL; light blue), brown bear from SWE (dark red), and black bear from Alaska (BLK; black). The European brown bear range is shown as dark gray (wild) and light gray (reintroduced in recent times). aRef. . bRef. . (B) Average heterozygosity per site (θW) in brown bear individuals sequenced at different depths. Genome sequences from two Swedish brown bears and one polar bear individual (* indicates that all 6 polar bears have very similar levels of variation) from previously published studies are also included. Analyses are also replicated in high-coverage individuals after down-sampling the raw reads (striped bars). (C) Long regions of homozygosity (>1 Mb with more than 99.95% of homozygous calls in contiguous 50-kb windows) in ascending length order. Note that brown bear (Upper) and polar bear (Lower) tracks are on different _x_- and _y_-axes scales. The area under each plot is proportional to the fraction of the genome (shown in parentheses) that can be assigned to these fragments. (D) Neighbor-joining tree using the whole-genome sequence (Left) or the whole-mitochondrial genome sequence (Right). The mitochondrial genome has also been sequenced in 11 additional samples (SI Appendix, section S6). Note that Swedish samples have two different mitochondrial haplotypes clustering with the Slovakian samples and the Spanish sample. (E) Examples of the genomic variation along >40 Mb of scaffold 1 in an Apennine brown bear individual (red), an Alpine brown bear (brown), and a polar bear (blue). Long windows of homozygosity in the Apennine sample are shaded in gray.
Fig. 2.
Inferred demographic history of the Apennine brown bear. (A) Demographic dynamics reconstructed applying the PSMC approach to the APN2 genome (solid line) with bootstrap-based uncertainty regions (shade). (B) Demographic dynamic of the Apennine brown bear estimated using the ABC skyline approach based on ∼20 Mb of noncoding regions from five individuals (median as a solid line; 95% confidence intervals are shaded). (C) Examples of patterns of the genomic distribution of θW per site in 1,000 fragments of 50 kb in 20 simulated individuals subjected to different bottlenecks (sketched, not to scale, in gray within each figure; width of the boxes is proportional to population size; time moves downwards). Gray lines represent each simulated individual, with θW values in ascending order, whereas red lines represent the pattern in the observed APN2 genome in 50 Mb of the longest scaffold (scaffold 1). _N_0 and _N_a correspond to the modern and the ancient population size, respectively, whereas Tb represents the time of the bottleneck (in years). The most supported scenario is reported in Upper, whereas three additional illustrative examples are reported in Lower (SI Appendix, section S3.3 and Fig. S10 has details and more examples).
Fig. 3.
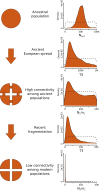
Inferred fragmentation scenario for the European brown bear. Posterior distributions in Right refer to the ABC metapopulation model (SI Appendix, section S5.2 has details); connectivity refers to Nm as the product of the effective population size of a deme, N, and the migration rate, m, per generation. _N_anc (in individuals) is the ancestral population size before any fragmentation. T5 (in years) is the time of brown bear spread in Europe. _N_1_m_1 is migration parameter among European brown bear populations before T3. T3 (in years) is the time of the decrease in connectivity among European brown bear populations. _N_2_m_2 is migration parameter among European brown bear populations after T3. Dashed lines represent prior distributions.
Fig. 4.
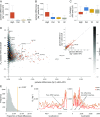
Active maintenance and random loss of genetic diversity. Boxplot of the average genetic diversity [estimated with ANGSD (74)] within five Apennine brown bears (A), within five European (EUR) brown bears (B), and between these two groups (C) per gene categories and including 10 kb upstream and downstream of each gene. The categories are as follows. High indicates genes in high-diversity genomic windows (SI Appendix, section S8.2). Del indicates genes with nonsynonymous deleterious mutations fixed in the Apennine population (SI Appendix, section S9.1). TA indicates candidate genes associated with TA behavior (SI Appendix, section S9.3). All indicates all of the other genes. (D) Scatterplot of genetic diversity within and between groups in single genes of different categories; gray shades correspond to different numbers of All genes with specific combinations of within and between differentiation. The most divergent gene for each category is indicated (High: RNPC3, Del: CMA1, and TA: GLTSCR2) as well as most of the genes with high diversity in the APN population: immune response (HLA and _IG_-like), carbohydrates digestion (AMY1B/2B), aflatoxin detoxification (AKR7A1), control of DNA damage (HUS1), lipid transporter activity (APOL2/3), and olfactory receptors (marked with asterisks). Note that some of the genes at high diversity in the APN population (e.g., APOL2/3) are not included in the High category, as they were excluded because of marginally overlapping TopWi windows with unusual coverage (SI Appendix, section S8.2). (E) Proportion of fixed differences in genes related to TA behavior is significantly higher (yellow line) than expected at random (gray bars). (F) Genomic windows in the 99th percentile (dashed lines) considering the genetic diversity in the European brown bear are also characterized by high diversity in the Apennine population and are enriched for immune system and olfactory receptor genes (brown shaded boxes). θW in the two groups along scaffold 118 and scaffold 80 is shown as an illustration. Positions of five MHC and six olfactory receptor genes are shown.
Similar articles
- Fitness consequences and ancestry loss in the Apennine brown bear after a simulated genetic rescue intervention.
Maroso F, Padovani G, Muñoz Mora VH, Giannelli F, Trucchi E, Bertorelle G. Maroso F, et al. Conserv Biol. 2023 Dec;37(6):e14133. doi: 10.1111/cobi.14133. Epub 2023 Sep 28. Conserv Biol. 2023. PMID: 37259604 - Analysis of complete mitochondrial genome sequences increases phylogenetic resolution of bears (Ursidae), a mammalian family that experienced rapid speciation.
Yu L, Li YW, Ryder OA, Zhang YP. Yu L, et al. BMC Evol Biol. 2007 Oct 24;7:198. doi: 10.1186/1471-2148-7-198. BMC Evol Biol. 2007. PMID: 17956639 Free PMC article. - MHC class II DQB diversity in the Japanese black bear, Ursus thibetanus japonicus.
Yasukochi Y, Kurosaki T, Yoneda M, Koike H, Satta Y. Yasukochi Y, et al. BMC Evol Biol. 2012 Nov 29;12:230. doi: 10.1186/1471-2148-12-230. BMC Evol Biol. 2012. PMID: 23190438 Free PMC article. - Impacts of Human Recreation on Brown Bears (Ursus arctos): A Review and New Management Tool.
Fortin JK, Rode KD, Hilderbrand GV, Wilder J, Farley S, Jorgensen C, Marcot BG. Fortin JK, et al. PLoS One. 2016 Jan 5;11(1):e0141983. doi: 10.1371/journal.pone.0141983. eCollection 2016. PLoS One. 2016. PMID: 26731652 Free PMC article. Review. - EPIDEMIOLOGIC AND PUBLIC HEALTH SIGNIFICANCE OF TOXOPLASMA GONDII INFECTIONS IN BEARS (URSUS SPP.): A 50 YEAR REVIEW INCLUDING RECENT GENETIC EVIDENCE.
Dubey JP, Murata FHA, Cerqueira-Cézar CK, Kwok OCH, Su C. Dubey JP, et al. J Parasitol. 2021 May 1;107(3):519-528. doi: 10.1645/21-16. J Parasitol. 2021. PMID: 34167147 Review.
Cited by
- Late Pleistocene polar bear genomes reveal the timing of allele fixation in key genes associated with Arctic adaptation.
Sun Y, Lorenzen ED, Westbury MV. Sun Y, et al. BMC Genomics. 2024 Sep 16;25(1):826. doi: 10.1186/s12864-024-10617-3. BMC Genomics. 2024. PMID: 39278943 Free PMC article. - Impact of supplementation on deleterious mutation distribution in an exploited salmonid.
Ferchaud AL, Laporte M, Perrier C, Bernatchez L. Ferchaud AL, et al. Evol Appl. 2018 Jul 1;11(7):1053-1065. doi: 10.1111/eva.12660. eCollection 2018 Aug. Evol Appl. 2018. PMID: 30026797 Free PMC article. - Conservation genomics provides insights into genetic resilience and adaptation of the endangered Chinese hazelnut, Corylus chinensis.
Yang Z, Liang L, Xiang W, Wang L, Ma Q, Wang Z. Yang Z, et al. Plant Divers. 2024 Apr 2;46(3):294-308. doi: 10.1016/j.pld.2024.03.006. eCollection 2024 May. Plant Divers. 2024. PMID: 38798732 Free PMC article. - Purging of Strongly Deleterious Mutations Explains Long-Term Persistence and Absence of Inbreeding Depression in Island Foxes.
Robinson JA, Brown C, Kim BY, Lohmueller KE, Wayne RK. Robinson JA, et al. Curr Biol. 2018 Nov 5;28(21):3487-3494.e4. doi: 10.1016/j.cub.2018.08.066. Epub 2018 Oct 25. Curr Biol. 2018. PMID: 30415705 Free PMC article. - Genetic architecture and evolution of color variation in American black bears.
Puckett EE, Davis IS, Harper DC, Wakamatsu K, Battu G, Belant JL, Beyer DE Jr, Carpenter C, Crupi AP, Davidson M, DePerno CS, Forman N, Fowler NL, Garshelis DL, Gould N, Gunther K, Haroldson M, Ito S, Kocka D, Lackey C, Leahy R, Lee-Roney C, Lewis T, Lutto A, McGowan K, Olfenbuttel C, Orlando M, Platt A, Pollard MD, Ramaker M, Reich H, Sajecki JL, Sell SK, Strules J, Thompson S, van Manen F, Whitman C, Williamson R, Winslow F, Kaelin CB, Marks MS, Barsh GS. Puckett EE, et al. Curr Biol. 2023 Jan 9;33(1):86-97.e10. doi: 10.1016/j.cub.2022.11.042. Epub 2022 Dec 16. Curr Biol. 2023. PMID: 36528024 Free PMC article.
References
- Charlesworth D, Willis JH. The genetics of inbreeding depression. Nat Rev Genet. 2009;10:783–796. - PubMed
- Lande R. Risk of population extinction from fixation of new deleterious mutations. Evolution. 1994;48:1460–1469. - PubMed
- Bürger R, Lynch M. Evolution and extinction in a changing environment: A quantitative-genetic analysis. Evolution. 1995;49:151–163. - PubMed
- Gilpin ME, Soulé ME. Minimum viable populations: Processes of species extinction. In: Soulé ME, editor. Conservation Biology: The Science of Scarcity and Diversity. Sinauer; Sunderland, MA: 1986. pp. 19–34.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources