A microtubule bestiary: structural diversity in tubulin polymers - PubMed (original) (raw)
Review
A microtubule bestiary: structural diversity in tubulin polymers
Sami Chaaban et al. Mol Biol Cell. 2017.
Abstract
Microtubules are long, slender polymers of αβ-tubulin found in all eukaryotic cells. Tubulins associate longitudinally to form protofilaments, and adjacent protofilaments associate laterally to form the microtubule. In the textbook view, microtubules are 1) composed of 13 protofilaments, 2) arranged in a radial array by the centrosome, and 3) built into the 9+2 axoneme. Although these canonical structures predominate in eukaryotes, microtubules with divergent protofilament numbers and higher-order microtubule assemblies have been discovered throughout the last century. Here we survey these noncanonical structures, from the 4-protofilament microtubules of Prosthecobacter to the 40-protofilament accessory microtubules of mantidfly sperm. We review the variety of protofilament numbers observed in different species, in different cells within the same species, and in different stages within the same cell. We describe the determinants of protofilament number, namely nucleation factors, tubulin isoforms, and posttranslational modifications. Finally, we speculate on the functional significance of these diverse polymers. Equipped with novel tubulin-purification tools, the field is now prepared to tackle the long-standing question of the evolutionary basis of microtubule structure.
© 2017 Chaaban and Brouhard. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures
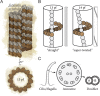
FIGURE 1:
(A) Microtubules are polymers of α/β-tubulin dimers typically composed of 13 protofilaments. (B) Adjacent protofilaments have a longitudinal offset of 9.2 Å between tubulins such that 13-protofilament microtubules have straight protofilaments (left). The lattice of non–13-protofilament microtubules (14 protofilaments shown) must accommodate by imposing a protofilament supertwist (right; not to scale). (C) The functional unit of cilia and flagella (left) is the 9+2 axoneme, with 2 central pair singlets and 9 outer microtubule doublets (middle). Doublets (right) are composed of an incomplete microtubule (10-protofilament B-tubule) clutched to the side of a complete 13-protofilament microtubule (A-tubule). pf, protofilament.
FIGURE 2:
A genus of bacteria, Prosthecobacter (left), is unique in its acquisition of tubulin-like genes that form bacterial microtubules (right, arrows; adapted from Pilhofer et al., 2011).
FIGURE 3:
(A) The nematode C. elegans (left) has diverged from the 13-protofilament microtubules observed in other eukaryotes, with 11-protofilament microtubules in ventral cord neurons (top right) and 15-protofilament microtubules in TRNs (bottom right; adapted from Chalfie and Thomson [1982] and reprinted with permission from Rockefeller University Press). (B) The crayfish Procambarus clarkii (left) has 12-protofilament microtubules in the neurons of the nerve cord (right), whereas supporting glial cells have 13-protofilament microtubules (not shown; adapted from Burton et al. [1975] and reprinted with permission from Rockefeller University Press). (C) The guinea pig Cavia porcellus (left) has bundles of 15-protofilament microtubules in its inner pillar cells (right; adapted from Saito and Hama [1982] and reprinted with permission from Oxford University Press). (D) Divergent protofilament numbers are also found in humans (left). A cross-section through a human blood platelet after treatment with 10 µM ADP (right) shows that microtubules sometimes have 14 protofilaments (top) as opposed to the standard 13 protofilaments (bottom; adapted from Xu and Afzelius [1988] and reprinted with permission from Elsevier).
FIGURE 4:
(A) Many insects, such as the caddisfly Stenophylax permistus (left), have unusual sperm axonemes with 9 accessory microtubules surrounding the 9 microtubule doublets in a 9+9+2 configuration (right; adapted from Dallai et al. [2016] and reprinted with permission from the Annual Review of Entomology). (B) The sperm axoneme accessory microtubules of the mantidfly M. perla have 40 protofilaments and are the largest microtubules observed in nature (adapted from Dallai et al. [2005] and reprinted with permission from Elsevier).
FIGURE 5:
(A) The γ-TuRC provides a template for nucleation with its 13 exposed γ-tubulins (Kollman et al., 2010; left). Shown is a 13-protofilament microtubule nucleated from the centrosome (right; adapted from Evans et al. [1985] and reprinted with permission from Rockefeller University Press). (B) The tubulin dimer (left) can be composed of different isoforms, such as the C. elegans MEC-12/MEC-7 (Savage et al., 1989; Fukushige et al., 1999), and can acquire PTMs, such as acetylation (Cueva et al., 2012; Topalidou et al., 2012). These modifications are able to specify the 15-protofilament microtubules of TRNs in C. elegans (right; adapted from Chalfie and Thomson [1982] and reprinted with permission from Rockefeller University Press).
FIGURE 6:
(A) A gall midge (left) has cartwheel arrangements of microtubule doublets in the axonemes of its sperm (right; adapted from Dallai et al. [1997] and reprinted with permission from John Wiley & Sons). (B) Heliozoans, such as A. nucleofilum (left), have thin extensions into the environment that contain two interlocking spiral sheets of microtubules that are subdivided into twelve sectors (right; adapted from Tilney and Byers [1969] and reprinted with permission from Rockefeller University Press).
Similar articles
- Posttranslational acetylation of α-tubulin constrains protofilament number in native microtubules.
Cueva JG, Hsin J, Huang KC, Goodman MB. Cueva JG, et al. Curr Biol. 2012 Jun 19;22(12):1066-74. doi: 10.1016/j.cub.2012.05.012. Epub 2012 May 31. Curr Biol. 2012. PMID: 22658592 Free PMC article. - Microtubules switch occasionally into unfavorable configurations during elongation.
Chrétien D, Fuller SD. Chrétien D, et al. J Mol Biol. 2000 May 12;298(4):663-76. doi: 10.1006/jmbi.2000.3696. J Mol Biol. 2000. PMID: 10788328 - Tipping microtubule dynamics, one protofilament at a time.
Aher A, Akhmanova A. Aher A, et al. Curr Opin Cell Biol. 2018 Feb;50:86-93. doi: 10.1016/j.ceb.2018.02.015. Epub 2018 Mar 21. Curr Opin Cell Biol. 2018. PMID: 29573640 Review. - How cells exploit tubulin diversity to build functional cellular microtubule mosaics.
Roll-Mecak A. Roll-Mecak A. Curr Opin Cell Biol. 2019 Feb;56:102-108. doi: 10.1016/j.ceb.2018.10.009. Epub 2018 Nov 20. Curr Opin Cell Biol. 2019. PMID: 30466050 Free PMC article. Review. - Structure of the γ-tubulin ring complex-capped microtubule.
Aher A, Urnavicius L, Xue A, Neselu K, Kapoor TM. Aher A, et al. Nat Struct Mol Biol. 2024 Jul;31(7):1124-1133. doi: 10.1038/s41594-024-01264-z. Epub 2024 Apr 12. Nat Struct Mol Biol. 2024. PMID: 38609661
Cited by
- Multi-curve fitting and tubulin-lattice signal removal for structure determination of large microtubule-based motors.
Chai P, Rao Q, Zhang K. Chai P, et al. J Struct Biol. 2022 Dec;214(4):107897. doi: 10.1016/j.jsb.2022.107897. Epub 2022 Sep 8. J Struct Biol. 2022. PMID: 36089228 Free PMC article. - Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles.
Wordeman L, Vicente JJ. Wordeman L, et al. Cancers (Basel). 2021 Nov 12;13(22):5650. doi: 10.3390/cancers13225650. Cancers (Basel). 2021. PMID: 34830812 Free PMC article. Review. - Effects of α-tubulin acetylation on microtubule structure and stability.
Eshun-Wilson L, Zhang R, Portran D, Nachury MV, Toso DB, Löhr T, Vendruscolo M, Bonomi M, Fraser JS, Nogales E. Eshun-Wilson L, et al. Proc Natl Acad Sci U S A. 2019 May 21;116(21):10366-10371. doi: 10.1073/pnas.1900441116. Epub 2019 May 9. Proc Natl Acad Sci U S A. 2019. PMID: 31072936 Free PMC article. - Structure-Guided Prediction of the Functional Impact of DCLK1 Mutations on Tumorigenesis.
Carli ALE, Hardy JM, Hoblos H, Ernst M, Lucet IS, Buchert M. Carli ALE, et al. Biomedicines. 2023 Mar 22;11(3):990. doi: 10.3390/biomedicines11030990. Biomedicines. 2023. PMID: 36979969 Free PMC article. Review. - [Significance of anti-tubulin-α-1C autoantibody in systemic sclerosis].
Zhao J, Sun F, Li Y, Zhao XZ, Xu D, Li YN, Li YH, Sun XL. Zhao J, et al. Beijing Da Xue Xue Bao Yi Xue Ban. 2020 Dec 18;52(6):1009-1013. doi: 10.19723/j.issn.1671-167X.2020.06.004. Beijing Da Xue Xue Bao Yi Xue Ban. 2020. PMID: 33331306 Free PMC article. Chinese.
References
- Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem. 2005;71:257–298. - PubMed
- Anand SP, Akhtar P, Tinsley E, Watkins SC, Khan SA. GTP-dependent polymerization of the tubulin-like RepX replication protein encoded by the pXO1 plasmid of Bacillus anthracis. Mol Microbiol. 2008;67:881–890. - PubMed
- Baccetti B, Burrini AG, Dallai R, Pallini V. A motile system of singlet microtubules in spermatozoa. Cell Motil. 1982;2:93–101.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources