Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told? - PubMed (original) (raw)
Review
Time to clinical response and remission for therapeutics in inflammatory bowel diseases: What should the clinician expect, what should patients be told?
Abhinav Vasudevan et al. World J Gastroenterol. 2017.
Abstract
An awareness of the expected time for therapies to induce symptomatic improvement and remission is necessary for determining the timing of follow-up, disease (re)assessment, and the duration to persist with therapies, yet this is seldom reported as an outcome in clinical trials. In this review, we explore the time to clinical response and remission of current therapies for inflammatory bowel disease (IBD) as well as medication, patient and disease related factors that may influence the time to clinical response. It appears that the time to therapeutic response varies depending on the indication for therapy (Crohn's disease or ulcerative colitis). Agents with the most rapid time to clinical response included corticosteroids, calcineurin inhibitors, exclusive enteral nutrition, aminosalicylates and anti-tumor necrosis factor therapy which will work in most patients within the first 2 mo. Vedolizumab, methotrexate and thiopurines had a longer time to clinical response and can take several months to achieve maximal efficacy. Factors affecting the time to clinical response of therapies included use of concomitant therapy, disease duration, smoking status, disease phenotype and advanced age. There appears to be marked variation in time to clinical response for therapies used in IBD which is further influenced by disease and patient related factors. Understanding the expected time to therapeutic response is integral to inform further decision making, maintain a patient-centered approach and ensure treatment is given an appropriate timeframe to achieve maximal benefit prior to cessation.
Keywords: Biologics; Clinical pharmacology; Crohn’s disease; Inflammatory bowel disease; Nutrition; Thiopurines; Ulcerative colitis.
Conflict of interest statement
Conflict-of-interest statement: Vasudevan A has no conflict of interest to declare; Gibson PR has served as consultant or advisory board member for AbbVie, Ferring, Janssen, Merck, Nestle Health Science, Danone, Allergan, Pfizer, Celgene and Takeda. His institution has received speaking honoraria from AbbVie, Janssen, Ferring, Takeda, Fresenius Kabi, Mylan and Pfizer. He has received research grants for investigator-driven studies from AbbVie, Janssen, Falk Pharma, Danone and A2 Milk Company. His Department financially benefits from the sales of a digital application and booklets on the low FODMAP diet. He has published an educational/recipe book on diet. van Langenberg DR has served as a speaker and/or received travel support from Takeda, Ferring and Shire. He has consultancy agreements with Abbvie, Janssen and Pfizer. He received research funding grants for investigator-driven studies from Ferring, Shire and AbbVie.
Figures
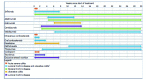
Figure 1
Schematic representation of the range of expected time of clinical response for therapies based on indication. 1This symbol is used to represent the expected time to response for therapies that can be used in either condition.
Similar articles
- Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease.
Renna S, Cottone M, Orlando A. Renna S, et al. World J Gastroenterol. 2014 Aug 7;20(29):9675-90. doi: 10.3748/wjg.v20.i29.9675. World J Gastroenterol. 2014. PMID: 25110407 Free PMC article. Review. - Use of Vedolizumab in Inflammatory Bowel Disease: A Single-Center Experience.
Erdoğan Ç, Yeşil B, Bacaksız F, Arı D, Gökbulut V, Yüksel M, Özderin Özin Y, Kayaçetin E. Erdoğan Ç, et al. Turk J Gastroenterol. 2022 Oct;33(10):831-837. doi: 10.5152/tjg.2022.21684. Turk J Gastroenterol. 2022. PMID: 35946879 Free PMC article. - Outcomes, dosing, and predictors of vedolizumab treatment in children with inflammatory bowel disease (VEDOKIDS): a prospective, multicentre cohort study.
Atia O, Shavit-Brunschwig Z, Mould DR, Stein R, Matar M, Aloi M, Ledder O, Focht G, Urlep D, Hyams J, Broide E, Weiss B, Levine J, Russell RK, Turner D. Atia O, et al. Lancet Gastroenterol Hepatol. 2023 Jan;8(1):31-42. doi: 10.1016/S2468-1253(22)00307-7. Epub 2022 Oct 26. Lancet Gastroenterol Hepatol. 2023. PMID: 36306803 - Considerations in Paediatric and Adolescent Inflammatory Bowel Disease.
Vuijk SA, Camman AE, de Ridder L. Vuijk SA, et al. J Crohns Colitis. 2024 Oct 30;18(Supplement_2):ii31-ii45. doi: 10.1093/ecco-jcc/jjae087. J Crohns Colitis. 2024. PMID: 39475081 Free PMC article. Review. - Review article: nutrition and adult inflammatory bowel disease.
Goh J, O'Morain CA. Goh J, et al. Aliment Pharmacol Ther. 2003 Feb;17(3):307-20. doi: 10.1046/j.1365-2036.2003.01482.x. Aliment Pharmacol Ther. 2003. PMID: 12562443 Review.
Cited by
- Highly multiplexed spatial analysis identifies tissue-resident memory T cells as drivers of ulcerative and immune checkpoint inhibitor colitis.
van Eijs MJM, Ter Linde JJM, Baars MJD, Amini M, Laclé MM, Brand EC, Delemarre EM, Drylewicz J, Nierkens S, Verheijden RJ, Oldenburg B, Vercoulen Y, Suijkerbuijk KPM, van Wijk F. van Eijs MJM, et al. iScience. 2023 Sep 11;26(10):107891. doi: 10.1016/j.isci.2023.107891. eCollection 2023 Oct 20. iScience. 2023. PMID: 37766980 Free PMC article. - Ulcerative Colitis: Current and Emerging Treatment Strategies.
Kayal M, Shah S. Kayal M, et al. J Clin Med. 2019 Dec 30;9(1):94. doi: 10.3390/jcm9010094. J Clin Med. 2019. PMID: 31905945 Free PMC article. Review. - Pharmacologic Management of Monogenic and Very Early Onset Inflammatory Bowel Diseases.
Levine AE, Mark D, Smith L, Zheng HB, Suskind DL. Levine AE, et al. Pharmaceutics. 2023 Mar 17;15(3):969. doi: 10.3390/pharmaceutics15030969. Pharmaceutics. 2023. PMID: 36986830 Free PMC article. Review. - Clinical Utility of Disease Activity Indices in Predicting Short-Term Response to Biologics in Patients with Ulcerative Colitis.
Romaniuk F, Franus A, Sobolewska-Włodarczyk A, Gąsiorowska A. Romaniuk F, et al. J Clin Med. 2024 Jun 13;13(12):3455. doi: 10.3390/jcm13123455. J Clin Med. 2024. PMID: 38929982 Free PMC article. - Multiple mesenteric lymphadenopathies in pediatric with ulcerative colitis: A case report.
Ratih SD, Makrufardi F, Azizah AFN, Damayanti W. Ratih SD, et al. Radiol Case Rep. 2023 Nov 24;19(2):600-603. doi: 10.1016/j.radcr.2023.10.046. eCollection 2024 Feb. Radiol Case Rep. 2023. PMID: 38074434 Free PMC article.
References
- Markowitz J. Early inflammatory bowel disease: different treatment response to specific or all medications? Dig Dis. 2009;27:358–365. - PubMed
- Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, Rene E. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Groupe d’Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990;98:811–818. - PubMed
- Cellier C, Sahmoud T, Froguel E, Adenis A, Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY, Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994;35:231–235. - PMC - PubMed
- Peyrin-Biroulet L, Reinisch W, Colombel JF, Mantzaris GJ, Kornbluth A, Diamond R, Rutgeerts P, Tang LK, Cornillie FJ, Sandborn WJ. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut. 2014;63:88–95. - PubMed
- Jharap B, Sandborn WJ, Reinisch W, D’Haens G, Robinson AM, Wang W, Huang B, Lazar A, Thakkar RB, Colombel JF. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther. 2015;42:1082–1092. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical