Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology - PubMed (original) (raw)
Review
. 2018 Jan 1;114(1):19-34.
doi: 10.1093/cvr/cvx211.
Sean Michael Davidson 2, Chantal M Boulanger 3, Edit Iren Buzás 4 5, Dominique Paschalis Victor de Kleijn 6 7, Felix Benedikt Engel 8, Zoltán Giricz 9, Derek J Hausenloy 10 11 12 13 14 15, Raj Kishore 16, Sandrine Lecour 17, Jonathan Leor 18, Rosalinda Madonna [ 19](#full-view-affiliation-19 "Center of Aging Science and Regenerative Medicine, CESI-Met and Institute of Cardiology, "G. D'Annunzio" University, Chieti-Pescara, Chieti, Italy.") 20 21, Cinzia Perrino 22, Fabrice Prunier 23, Susmita Sahoo 24, Ray Michel Schiffelers 25, Rainer Schulz 26, Linda Wilhelmina Van Laake 27, Kirsti Ytrehus 28, Péter Ferdinandy 29 30
Affiliations
- PMID: 29106545
- PMCID: PMC5852624
- DOI: 10.1093/cvr/cvx211
Review
Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology
Joost Petrus Gerardus Sluijter et al. Cardiovasc Res. 2018.
Abstract
Extracellular vesicles (EVs)-particularly exosomes and microvesicles (MVs)-are attracting considerable interest in the cardiovascular field as the wide range of their functions is recognized. These capabilities include transporting regulatory molecules including different RNA species, lipids, and proteins through the extracellular space including blood and delivering these cargos to recipient cells to modify cellular activity. EVs powerfully stimulate angiogenesis, and can protect the heart against myocardial infarction. They also appear to mediate some of the paracrine effects of cells, and have therefore been proposed as a potential alternative to cell-based regenerative therapies. Moreover, EVs of different sources may be useful biomarkers of cardiovascular disease identities. However, the methods used for the detection and isolation of EVs have several limitations and vary widely between studies, leading to uncertainties regarding the exact population of EVs studied and how to interpret the data. The number of publications in the exosome and MV field has been increasing exponentially in recent years and, therefore, in this ESC Working Group Position Paper, the overall objective is to provide a set of recommendations for the analysis and translational application of EVs focussing on the diagnosis and therapy of the ischaemic heart. This should help to ensure that the data from emerging studies are robust and repeatable, and optimize the pathway towards the diagnostic and therapeutic use of EVs in clinical studies for patient benefit.
Keywords: Cardioprotection; Co-morbidities; Exosomes; Extracellular vesicles; Heart failure; Ischaemia; Microvesicles; Postconditioning; Preconditioning; Regenerative medicine; Remote conditioning; Reperfusion.
© The Author 2017. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
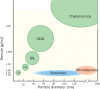
Figure 1
Exosomes and MVs overlap in size with VLDL and chylomicrons, and in density with HDL/LDL particles. Exosome density is typically 1.06–1.20 g/mL. MV density is not well defined but they have been found between ∼1.03–1.08 g/mL.
Figure 2
The major classes of EV. Typical size and density of EV classes and some of the contaminants that may be co-isolated, depending on biofluid.
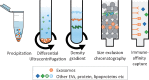
Figure 3
Standard techniques used for isolating exosomes from other EVs, protein, and lipoproteins present in blood and cell-culture medium.
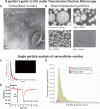
Figure 4
Standard methods of characterizing EVs. (A) Transmission electron microscopy (TEM) of negative-stained EVs reveals the ‘cup-shaped’ appearance of exosomes (E) and MVs once they have been dried for TEM (they are spherical in solution). (B) The spherical appearance of lipoprotein particles by TEM is quite distinct (image courtesy of Robert L. Hamilton and the Arteriosclerosis Specialized Center of Research, University of California, San Francisco). (C) Nanoparticle tracking analysis (NTA) provides a size distribution of particles based on calculating their size by their random Brownian motion. (D, E) Tuneable resistance pulse sensing (TRP) determines size distribution by the change in resistance as the particle crosses a small pore in a membrane (which is selected according to the size range examined).
References
- Lawson C, Vicencio JM, Yellon DM, Davidson SM.. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 2016;228:R57–R71. - PubMed
- Barile L, Moccetti T, Marbán E, Vassalli G.. Roles of exosomes in cardioprotection. Eur Heart J 2017;38:1372–1379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FS/10/039/28270/BHF_/British Heart Foundation/United Kingdom
- P01 HL134608/HL/NHLBI NIH HHS/United States
- R01 HL126186/HL/NHLBI NIH HHS/United States
- MR/K002066/1/MRC_/Medical Research Council/United Kingdom
- R01 HL091983/HL/NHLBI NIH HHS/United States
- PG/16/85/32471 /BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical