Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide - PubMed (original) (raw)
doi: 10.1038/s41598-017-14709-x.
Lorena Simón-Gracia 3, Sergei Kopanchuk 4, Allan Tobi 3, Kalle Kilk 5, Pille Säälik 3, Kaarel Kurm 3, Mario Leonardo Squadrito 6, Venkata Ramana Kotamraju 7, Ago Rinken 4, Michele De Palma 6, Erkki Ruoslahti 7 8, Tambet Teesalu 9 10 11
Affiliations
- PMID: 29116108
- PMCID: PMC5676682
- DOI: 10.1038/s41598-017-14709-x
Precision Targeting of Tumor Macrophages with a CD206 Binding Peptide
Pablo Scodeller et al. Sci Rep. 2017.
Abstract
Tumor-associated macrophages (TAMs) expressing the multi-ligand endocytic receptor mannose receptor (CD206/MRC1) contribute to tumor immunosuppression, angiogenesis, metastasis, and relapse. Here, we describe a peptide that selectively targets MRC1-expressing TAMs (MEMs). We performed in vivo peptide phage display screens in mice bearing 4T1 metastatic breast tumors to identify peptides that target peritoneal macrophages. Deep sequencing of the peptide-encoding inserts in the selected phage pool revealed enrichment of the peptide CSPGAKVRC (codenamed "UNO"). Intravenously injected FAM-labeled UNO (FAM-UNO) homed to tumor and sentinel lymph node MEMs in different cancer models: 4T1 and MCF-7 breast carcinoma, B16F10 melanoma, WT-GBM glioma and MKN45-P gastric carcinoma. Fluorescence anisotropy assay showed that FAM-UNO interacts with recombinant CD206 when subjected to reducing conditions. Interestingly, the GSPGAK motif is present in all CD206-binding collagens. FAM-UNO was able to transport drug-loaded nanoparticles into MEMs, whereas particles without the peptide were not taken up by MEMs. In ex vivo organ imaging, FAM-UNO showed significantly higher accumulation in sentinel lymph nodes than a control peptide. This study suggests applications for UNO peptide in diagnostic imaging and therapeutic targeting of MEMs in solid tumors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
Identification of CSPGAKVRC (“UNO”) in breast cancer mice. (a) Naïve phage library was injected intraperitoneally in 4T1 tumor-bearing mice and age-matched normal mice, and allowed to circulate for 2 h. Peritoneal cells were collected, the accompanying phages were rescued and the peptide-encoding segment of phage DNA was sequenced. (b) Higher number of CD206+ cells were seen in the 4T1 mice than normal mice. Peritoneal cells were extracted from the mice, seeded on coverslips, allowed to attach for 2 h, fixed, permeabilized, and stained for CD206. CD206+ cells were counted from 6 different confocal images, 2 from each mouse. (c) Highly repeated sequences obtained from the first round of the biopanning experiment are shown schematically in panel A. (d) Frequency of phage clones encoding UNO or a randomly picked peptide (CIGVSSDC) divided by the total number of sequences) in the 4T1 tumor-bearing and normal mice.
Figure 2
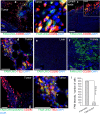
FAM-UNO accumulates in CD206+, TIE2+ macrophages in breast tumors and lymph nodes. Thirty nmoles of FAM-UNO or FAM-CRKQGEAKC control peptide were injected intravenously into 4T1 tumor-bearing mice and allowed to circulate for 2 h. Mice were then sacrificed and tumor and tissues were analyzed by immunofluorescence using rabbit anti-FAM (green) and rat anti-CD206 or rat anti-TIE2 (red) antibodies, and counterstained with DAPI. All images were taken under the same imaging conditions. FAM-UNO accumulated in macrophages within tumors and lymph nodes positive for CD206 staining (a–c) and TIE2 (d, and h: Blow up of d). FAM-UNO showed very low accumulation in the liver (e), but signal was seen in the kidneys (f), which is the normal excretion route for peptides. No signal or only traces of FAM-UNO were observed in the spleen, heart and lungs in images taken under the same conditions (shown in Fig. S4). To ascertain that anti-CD206 is capable of detecting CD206 in the liver, an image was acquired with higher gain from an uninjected animal (Fig. S19). The charge-matched control peptide, CRKQGEAKC, did not give any signal in CD206+ macrophages or elsewhere in the tumor (g and i). Green: FAM-peptide; Red: CD206 or TIE2, Blue: DAPI. Representative fields from multiple sections prepared from at least 3 tumors (n ≥ 3 mice) are shown. The graph in panel I shows mean + SEM of FAM signal quantified as described in materials and methods. Scale bars: 100 µm for a, b, c, e, f, g and 50 µm for d and h.
Figure 3
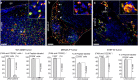
FAM-UNO accumulates in CD206+ macrophages in glioblastoma, gastric carcinoma, and melanoma. Thirty nmoles of FAM-UNO or FAM-control peptide were injected intravenously into mice and allowed to circulate for 2 h. Mice were then sacrificed and tumor and tissues were analyzed by immunofluorescence using rabbit anti-FAM (green) and rat anti-CD206 (red) antibodies, and counterstained with DAPI. (a) Homing to glioblastoma (WT-GBM). Signal was seen exclusively within the tumor (T) and not in the brain parenchyma (BP). (b) Homing to a peritoneal carcinomatosis lesion (PCL) induced by i.p. inoculation of gastric carcinoma cell line MKN4-5P. (c) Homing to experimental melanoma metastases in the lungs. The metastases were induced by i.v. inoculation of B16F10 melanoma cells. FAM signal was seen in lung metastases (LMM), and not in noncancerous lung parenchyma (LP). The arrows point to examples of FAM and CD206 colocalization in each panel. The insets show CD206-positive individual cells with internalized FAM-UNO signal. A parallel experiment with the control peptide (FAM-Control) is shown in Fig. S9A–C; images of spleens from mice injected with FAM-UNO are shown in Fig. S13. Blue: DAPI. Representative fields from multiple sections (n ≥ 3) prepared from at least 3 tumors are shown. Scale bar: 50 µm. Images shown are representative fields from multiple sections (≥3) prepared from at least 3 tumors (n ≥ 3 mice). The graphs show mean + SEM of FAM signal quantified as described in Materials and Methods.
Figure 4
UNO specificity for CD206. (a) Change in fluorescence anisotropy of FAM-UNO (dotted line) and FAM-UNO in DTT (solid line) while incubating with mouse recombinant CD206. (b) Change in fluorescence anisotropy of FAM-CSPGAK with mouse recombinant CD206 (solid line) or with CD163 (dotted line) and of FAM-CPMTDNE (control) with CD206 (dashed line). (c) FAM-UNO binds selectively to CCR2+ macrophages collected from the peritoneal cavity of 4T1 tumor-bearing mice, as 94.3% of FAM+ cells are CCR2+ cells. The analysis was done gating for the FAM+ population (left panel). In these 4T1 tumor bearing mice, 58% of peritoneal cells are macrophages, i.e CCR2+ cells (right panel). (d) FAM-UNO binding to peritoneal cells is inhibited by preincubating with 10 µg/mL of anti-CD206, whereas the preincubation with anti CD206 antibody had no effect on FAM-LyP-1 binding. In panels a and d are shown representative graphs from three independent experiments. In panels c and d are shown results from three independent experiments (n = 3 mice) and bars of panel d represents mean + SEM.
Figure 5
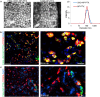
(a) Characterization of FAM-UNO derivatized, paclitaxel loaded, polymeric vesicles (“FAM-UNO-NP-PTX”). Transmission electron microscopy images (two panels on the left, scale bar: 200 nm) and Dynamic Light Scattering profile (right panel). Polymeric vesicles are composed of the copolymer polyethylene glycol-polycaprolactone. (b,c) FAM-UNO guides cargo-loaded nanoparticles inside MEMs. FAM-UNO-NP-PTX were intravenously injected in mice bearing MCF-7 tumors, 21 days after orthotopic inoculation of 5 × 106 cells. The particles were allowed to circulate for 6 h. The mice were then sacrificed, and tumors and tissues were analyzed by immunofluorescence using rabbit anti-FAM (green) and rat anti-CD206 (red) antibodies and counterstained with DAPI (blue). Images shown are representative fields from multiple sections (≥3) prepared from 3 tumors (n = 3 mice).
Figure 6
FAM-UNO can be used to image metastasis-draining lymph nodes. (a) FAM-UNO and FAM-LyP-1 were injected i.p at doses of 30nmoles in 4T1 tumor-bearing mice. Peptides were allowed to circulate for two h, mice were then sacrificed, and the organs were collected and imaged with the live imaging system MX3 Art Optix in the FITC channel with laser excitation. (b) The signal in each organ was quantified, normalized to the tissue weight and the ratio tissue/kidney was graphed in bar graph. LyP-1 is a peptide that targets p32 protein on the surface of activated macrophages,. Results from n = 3 mice. Bars of panel B represent mean + SEM.
Similar articles
- Targeting Pro-Tumoral Macrophages in Early Primary and Metastatic Breast Tumors with the CD206-Binding mUNO Peptide.
Lepland A, Asciutto EK, Malfanti A, Simón-Gracia L, Sidorenko V, Vicent MJ, Teesalu T, Scodeller P. Lepland A, et al. Mol Pharm. 2020 Jul 6;17(7):2518-2531. doi: 10.1021/acs.molpharmaceut.0c00226. Epub 2020 Jun 1. Mol Pharm. 2020. PMID: 32421341 - Phage-Display-Derived Peptide Binds to Human CD206 and Modeling Reveals a New Binding Site on the Receptor.
Asciutto EK, Kopanchuk S, Lepland A, Simón-Gracia L, Aleman C, Teesalu T, Scodeller P. Asciutto EK, et al. J Phys Chem B. 2019 Mar 7;123(9):1973-1982. doi: 10.1021/acs.jpcb.8b11876. Epub 2019 Feb 27. J Phys Chem B. 2019. PMID: 30768279 - Molecular imaging of tumor-infiltrating macrophages in a preclinical mouse model of breast cancer.
Sun X, Gao D, Gao L, Zhang C, Yu X, Jia B, Wang F, Liu Z. Sun X, et al. Theranostics. 2015 Feb 27;5(6):597-608. doi: 10.7150/thno.11546. eCollection 2015. Theranostics. 2015. PMID: 25825599 Free PMC article. - Exploiting Manipulated Small Extracellular Vesicles to Subvert Immunosuppression at the Tumor Microenvironment through Mannose Receptor/CD206 Targeting.
Fiani ML, Barreca V, Sargiacomo M, Ferrantelli F, Manfredi F, Federico M. Fiani ML, et al. Int J Mol Sci. 2020 Aug 31;21(17):6318. doi: 10.3390/ijms21176318. Int J Mol Sci. 2020. PMID: 32878276 Free PMC article. Review. - Mannosylated Constructs as a Platform for Cell-Specific Delivery of Bioactive Agents.
Tiwari S. Tiwari S. Crit Rev Ther Drug Carrier Syst. 2018;35(2):157-194. doi: 10.1615/CritRevTherDrugCarrierSyst.2018020313. Crit Rev Ther Drug Carrier Syst. 2018. PMID: 29717665 Review.
Cited by
- Clinical Translation of [68Ga]Ga-NOTA-anti-MMR-sdAb for PET/CT Imaging of Protumorigenic Macrophages.
Xavier C, Blykers A, Laoui D, Bolli E, Vaneyken I, Bridoux J, Baudhuin H, Raes G, Everaert H, Movahedi K, Van Ginderachter JA, Devoogdt N, Caveliers V, Lahoutte T, Keyaerts M. Xavier C, et al. Mol Imaging Biol. 2019 Oct;21(5):898-906. doi: 10.1007/s11307-018-01302-5. Mol Imaging Biol. 2019. PMID: 30671739 - Immunosuppression and outcomes in adult patients with de novo acute myeloid leukemia with normal karyotypes.
Ferraro F, Miller CA, Christensen KA, Helton NM, O'Laughlin M, Fronick CC, Fulton RS, Kohlschmidt J, Eisfeld AK, Bloomfield CD, Ramakrishnan SM, Day RB, Wartman LD, Uy GL, Welch JS, Christopher MJ, Heath SE, Baty JD, Schuelke MJ, Payton JE, Spencer DH, Rettig MP, Link DC, Walter MJ, Westervelt P, DiPersio JF, Ley TJ. Ferraro F, et al. Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2116427118. doi: 10.1073/pnas.2116427118. Proc Natl Acad Sci U S A. 2021. PMID: 34845035 Free PMC article. - Peptide-based targeting of immunosuppressive cells in cancer.
Trac NT, Chung EJ. Trac NT, et al. Bioact Mater. 2020 Jan 15;5(1):92-101. doi: 10.1016/j.bioactmat.2020.01.006. eCollection 2020 Mar. Bioact Mater. 2020. PMID: 31956738 Free PMC article. Review. - Targeting Tumors Using Peptides.
Scodeller P, Asciutto EK. Scodeller P, et al. Molecules. 2020 Feb 13;25(4):808. doi: 10.3390/molecules25040808. Molecules. 2020. PMID: 32069856 Free PMC article. Review. - Novel Anthracycline Utorubicin for Cancer Therapy.
Simón-Gracia L, Sidorenko V, Uustare A, Ogibalov I, Tasa A, Tshubrik O, Teesalu T. Simón-Gracia L, et al. Angew Chem Int Ed Engl. 2021 Jul 26;60(31):17018-17027. doi: 10.1002/anie.202016421. Epub 2021 Jun 1. Angew Chem Int Ed Engl. 2021. PMID: 33908690 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous