Sequence analysis and characterization of pyruvate kinase from Clonorchis sinensis, a 53.1-kDa homopentamer, implicated immune protective efficacy against clonorchiasis - PubMed (original) (raw)
doi: 10.1186/s13071-017-2494-9.
Tingjin Chen 1 2 3, Hongye Jiang 1 2 3, Hengchang Sun 1 2 3, Pengli Ren 5, Lu Zhao 1 2 3, Huimin Dong 1 2 3 4, Mengchen Shi 1 2 3, Zhiyue Lv 1 2 3, Zhongdao Wu 1 2 3, Xuerong Li 1 2 3, Xinbing Yu 1 2 3, Yan Huang 6 7 8, Jin Xu 9 10 11
Affiliations
- PMID: 29121987
- PMCID: PMC5680780
- DOI: 10.1186/s13071-017-2494-9
Sequence analysis and characterization of pyruvate kinase from Clonorchis sinensis, a 53.1-kDa homopentamer, implicated immune protective efficacy against clonorchiasis
Tingjin Chen et al. Parasit Vectors. 2017.
Abstract
Background: Clonorchis sinensis, the causative agent of clonorchiasis, is classified as one of the most neglected tropical diseases and affects more than 15 million people globally. This hepatobiliary disease is highly associated with cholangiocarcinoma. As key molecules in the infectivity and subsistence of trematodes, glycolytic enzymes have been targets for drug and vaccine development. Clonorchis sinensis pyruvate kinase (CsPK), a crucial glycolytic enzyme, was characterized in this research.
Results: Differences were observed in the sequences and spatial structures of CsPK and PKs from humans, rats, mice and rabbits. CsPK possessed a characteristic active site signature (IKLIAKIENHEGV) and some unique sites but lacked the N-terminal domain. The predicted subunit molecular mass (Mr) of CsPK was 53.1 kDa. Recombinant CsPK (rCsPK) was a homopentamer with a Mr. of approximately 290 kDa by both native PAGE and gel filtration chromatography. Significant differences in the protein and mRNA levels of CsPK were observed among four life stages of C. sinensis (egg, adult worm, excysted metacercaria and metacercaria), suggesting that these developmental stages may be associated with diverse energy demands. CsPK was widely distributed in adult worms. Moreover, an intense Th1-biased immune response was persistently elicited in rats immunized with rCsPK. Also, rat anti-rCsPK sera suppressed C. sinensis adult subsistence both in vivo and in vitro.
Conclusions: The sequences and spatial structures, molecular mass, and expression profile of CsPK have been characterized. rCsPK was indicated to be a homopentamer. Rat anti-rCsPK sera suppressed C. sinensis adult subsistence both in vivo and in vitro. CsPK is worthy of further study as a promising target for drug and vaccine development.
Keywords: Clonorchis sinensis; Drug target; Excretory/secretory products; Expression profile; Immune response; Pentamer; Pyruvate kinase; Vaccine candidate.
Conflict of interest statement
Ethics approval and consent to participate
SD rats and BALB/c mice were purchased from the animal centre of Sun Yat-sen University and raised carefully according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental procedures were authorized by the Animal Care and Use Committee of Sun Yat-sen University (Permit Numbers: SCXK (Guangdong) 2010–0107).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Fig. 1
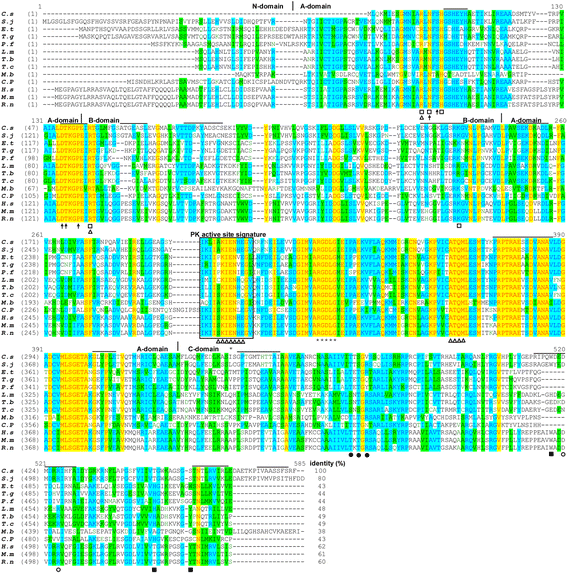
Sequence analysis of _Cs_PK. An alignment of the protein sequence of _Cs_PK with those of PKs from other organisms is shown. Clonorchis sinensis (C.s, GAA54498.1), Schistosoma japonicum (S.j, AAW27129.1), Eimeria tenella (E.t, AAC02529.1), Toxoplasma gondii (T.g, BAB47171.1), Plasmodium falciparum (P.f, CAD50538.1), Leishmania mexicana (L.m, CAA52898.2), Trypanosoma brucei (T.b, P30615.1), Trypanosoma cruzi (T.c, EKG02834.1), Mastigamoeba balamuthi (M.b, AAK94944.1), Cryptosporidium parvum (C.p, 4DRS_A), Homo sapiens (H.s, AAA60104.1), Mus musculus (M.m, NP_001093249.1), and Rattus norvegicus (R.n, AAA41880.1). The 3D–domains (N/A/B/C) are marked with vertical lines. The 22 predicted B cell linear epitopes with more trustworthiness are indicated by the black lines above the alignment. The rectangle indicates the PK active site signature. The triangles and open squares indicate the PEP and ADP binding sites, respectively. The binding sites of the sugar, 1-phosphate and 6-phosphate moieties of the allosteric activator fructose-1,6-bisphosphate (F16BP) are indicated by closed squares, open circles and closed circles, respectively. The black arrows and asterisks indicate monovalent cation and divalent cation binding sites, respectively
Fig. 2
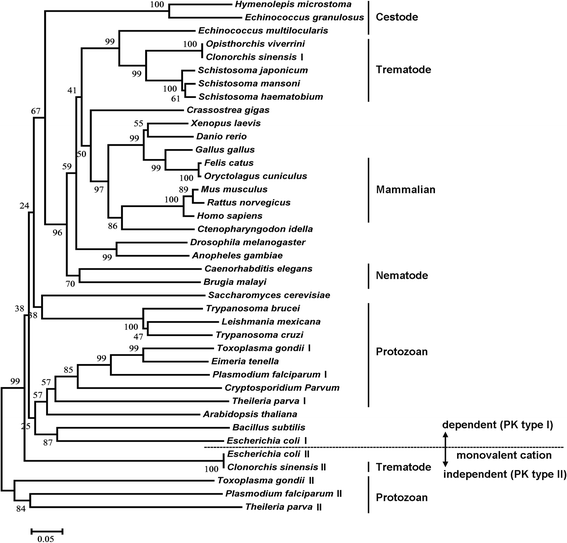
Neighbour-joining phylogenetic tree of PKs. The bootstrap values are displayed at the branching point (test of phylogeny by the bootstrap method with 1000 replications). The bar indicates the substitution by p-distance method. The protein sequences were obtained from GenBank and DDBJ. The sequences are as follows: Escherichia coli (AAA24392.1, AAA24473.1), Bacillus subtilis (P80885.2), Arabidopsis thaliana (BAB10461.1), Cryptosporidium parvum (4DRS_A), Plasmodium falciparum (CAD50538.1, AAN35560), Eimeria tenella (AAC02529.1), Toxoplasma gondii (BAB47171.1, KFH06835.1), Trypanosoma brucei (P30615.1), Trypanosoma cruzi (EKG02834.1), Leishmania mexicana (CAA52898.2), Theileria parva (XP_764242.1, XP_764703.1), Saccharomyces cerevisiae (CAA24631.1), Brugia malayi (XP_001898626.1), Hymenolepis microstoma (CDS33796.1), Echinococcus granulosus (CDS23463.1), Echinococcus multilocularis (CDS43052.1), Caenorhabditis elegans (CAA93424.2), Anopheles gambiae (EAA10555.6), Drosophila melanogaster (AAC16244.1), Crassostrea gigas (CAJ28914.1), Clonorchis sinensis (GAA54498.1, GAA58090.1), Opisthorchis viverrini (KER20867.1), Schistosoma japonicum (AAW27129.1), Schistosoma haematobium (KGB40466.1), Schistosoma mansoni (CCD76479.1), Danio rerio (NP_955365.1), Xenopus laevis (NP_001084341.1), Gallus gallus (NP_990800.1), Felis catus (P11979.2), Homo sapiens (AAA60104.1), Mus musculus (NP_001093249.1), Rattus norvegicus (AAA41880.1), Ctenopharyngodon idella (AFY98078.1)
Fig. 3
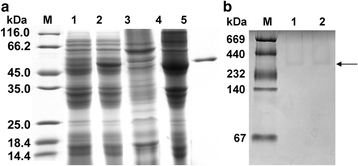
Expression and apparent Mr. of r_Cs_PK. Proteins were visualized by Coomassie Blue staining. Lane M contains protein molecular weight markers. a Expression and purification of r_Cs_PK. Lysate of E. coli transformed with pET-28a(+)-_Cs_PK without induction (Lane 1) and with induction (Lane 2); supernatant (Lane 3) and precipitate (Lane 4) of lysate of E. coli with pET-28a(+)-_Cs_PK with induction; and purified r_Cs_PK (Lane 5). b In Lane M, the protein bands with known Mr. (in descending order) are thyroglobulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), lactate dehydrogenase (140 kDa), and BSA (67 kDa). Lane 1, Freshly purified r_Cs_PK; Lane 2, r_Cs_PK stored for 4 weeks at -80 °C with 4 cycles of freezing and thawing
Fig. 4
Western blotting of r_Cs_PK after SDS-PAGE. Blots containing r_Cs_PK were incubated with preimmune mouse serum (Lane 1), a mouse His-tagged monoclonal antibody (Lane 2), mouse anti-r_Cs_PK sera (Lane 3), sera from mice infected with C. sinensis (Lane 4), or mouse anti-_Cs_ESPs sera (Lane 5). Blots containing _Cs_ESPs were incubated with mouse anti-r_Cs_PK sera (Lane 6) or with preimmune mouse serum (Lane 7). Blots containing total worm extract were incubated with mouse anti-r_Cs_PK sera (Lane 8) or with preimmune mouse serum (Lane 9)
Fig. 5
mRNA and protein levels of _Cs_PK at various life stages of C. sinensis. a Real- time PCR. The β-actin mRNA of C. sinensis was used as an internal control. Semiquantitative analysis was conducted using the 2-ΔΔCt method. Significant differences in the mRNA levels of _Cs_PK in egg, adult, excysted metacercaria, and metacercaria were observed (P < 0.01). The mRNA level of _Cs_PK in egg was higher than that in adult (56.79-fold, t (2) = 17.392, P = 0.003), metacercaria (9.72-fold, t (2.02) = 15.844, P = 0.004) and excysted metacercaria (5.97-fold, t (4) = 14.477, P < 0.001). b Western blotting. Total protein (40 μg) in extracts obtained at each life stage was probed with mouse anti-r_Cs_PK sera, revealing specific immunoreactive protein bands at approximately 53.1 kDa. No corresponding band was detected with preimmune mouse serum (data not shown). c Relative protein levels were analysed using Tanon Gis software. The protein level of _Cs_PK was maximal in eggs, followed by excysted metacercaria, metacercaria, and adults. The protein levels were consistent with the mRNA levels. (*P < 0.05; **P < 0.01; egg vs adult: t (4) = 12.950, P < 0.001; excysted metacercaria vs adult: t (4) = 16.542, P < 0.001; metacercaria vs adult: t (4) = 13.951, P < 0.001; excysted metacercaria vs metacercaria: t (4) = -3.680, P = 0.021)
Fig. 6
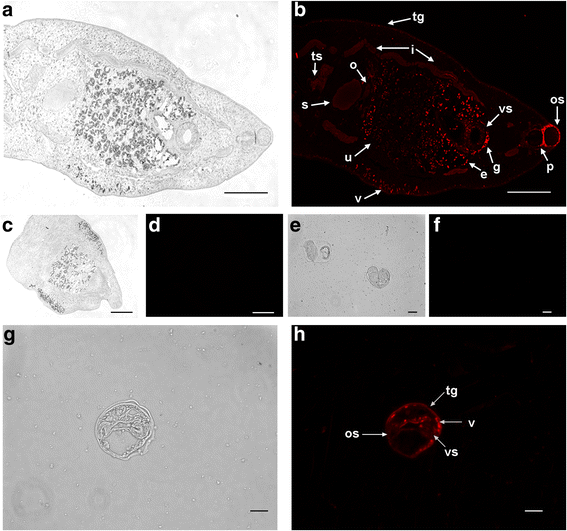
Immunolocalization of _Cs_PK in C. sinensis. Mouse anti-r_Cs_PK sera and Cy3-conjugated goat anti-mouse IgG were used as the primary and secondary antibodies, respectively. Preimmune mouse serum was used as the primary antibody for the negative controls. Panels (c), (d), (e), and (f) show negative controls. Panels (b), (d), (f), and h are fluorescence microscopic images; the same areas of the samples photographed under white light are shown in panels (a), (c), (e), and g with scale-bars. Panel (b), localization of _Cs_Pk in adults; panel h, localization of _Cs_Pk in metacercariae. Abbreviations: tg, tegument; e, egg; v, vitellarium; os, oral sucker; vs, ventral sucker; g, genital pore; s, seminal receptacle; i, intestine; ts, testicle; u, uterus;o, ovary; p, pharynx. Scale-bars: a-d, 100 μm; e-h, 10 μm
Fig. 7
ELISA determination of antibody titres and isotypes of IgG elicited by r_Cs_PK. Antibody titres of IgG elicited by r_Cs_PK in rats (a) and mice (b). IgG isotypes elicited by r_Cs_PK in rats (c). *P ≦ 0.001. 2 week: t (6) = 6.886, P < 0.001; 4 week: t (6) = 27.959, P < 0.001; 6 week: t (6) = 19.829, P < 0.001; 8 week: t (6) = 19.278, P < 0.001; 10 week: t (6) = 6.264, P = 0.001; 12 week: t (6) = 17.319, P < 0.001; 14 week: t (6) = 16.977, P < 0.001; 16 week: t (6) = 15.057, P < 0.001; 18 week: t (6) = 37.271, P < 0.001; 20 week: t (6) = 48.557, P < 0.001; 22 week: t (6) = 40.796, P < 0.001; 24 week: t (6) = 32.550, P < 0.001
Fig. 8
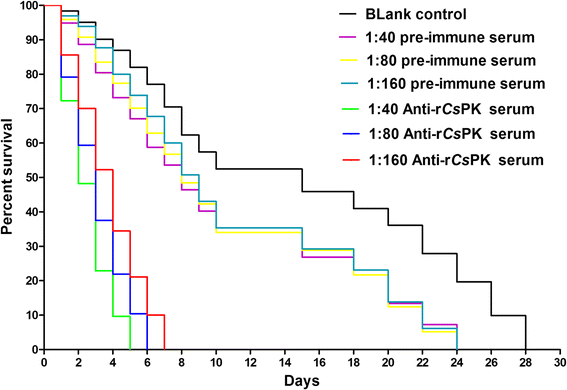
Rat anti-r_Cs_PK sera inhibits C. sinensis adult subsistence in vitro. The median subsistence times of C. sinensis adults in the blank control group, the 1:40 preimmune serum group, the 1:80 preimmune serum group, the 1:160 preimmune serum group, the 1:40 anti-r_Cs_PK serum group, the 1:80 anti-r_Cs_PK serum group, and the 1:160 anti-r_Cs_PK serum group were 15, 8, 8, 9, 2, 3 and 4 days, respectively. No significant difference in the rate of survival of the preimmune serum groups was observed at any serum dilution (1:40 preimmune serum group vs 1:80 preimmune serum group: χ 2 = 0.01289, df = 1, P = 0.9096; 1:80 preimmune serum group vs 1:160 preimmune serum group: χ 2 = 0.09872, df = 1, P = 0.7534; 1:40 preimmune serum group vs 1:160 preimmune serum group: χ 2 = 0.1657, df = 1, P = 0.6839). There were significant differences among the other groups in the rate of survival (1:40 anti-r_Cs_PK serum group vs 1:80 anti-r_Cs_PK serum group: χ 2 = 8.058, df = 1, P = 0.0045; 1:80 anti-r_Cs_PK serum group vs 1:160 anti-r_Cs_PK serum group: χ 2 = 8.092, df = 1, P = 0.0044; blank control group vs 1:40 preimmune serum group: χ 2 = 16.15, df = 1, P < 0.0001; blank control group vs 1:80 preimmune serum group: χ 2 = 15.54, df = 1, P < 0.0001; blank control group vs 1:160 preimmune serum group: χ 2 = 11.39, df = 1, P = 0.0007)
References
MeSH terms
Substances
Grants and funding
- 2017YFD0501300/the national key research and development program of China
- 2016A050502008/the Science and Technology Planning Project of Guangdong Province
- 16ykpy06/the Young Teachers' Cultivation Project of Basic Scientific Research Service Fee in Colleges and Universities
- 81772212/the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous