Transport of drugs from blood vessels to tumour tissue - PubMed (original) (raw)
Review
. 2017 Dec;17(12):738-750.
doi: 10.1038/nrc.2017.93. Epub 2017 Nov 10.
Affiliations
- PMID: 29123246
- PMCID: PMC6371795
- DOI: 10.1038/nrc.2017.93
Review
Transport of drugs from blood vessels to tumour tissue
Mark W Dewhirst et al. Nat Rev Cancer. 2017 Dec.
Abstract
The effectiveness of anticancer drugs in treating a solid tumour is dependent on delivery of the drug to virtually all cancer cells in the tumour. The distribution of drug in tumour tissue depends on the plasma pharmacokinetics, the structure and function of the tumour vasculature and the transport properties of the drug as it moves through microvessel walls and in the extravascular tissue. The aim of this Review is to provide a broad, balanced perspective on the current understanding of drug transport to tumour cells and on the progress in developing methods to enhance drug delivery. First, the fundamental processes of solute transport in blood and tissue by convection and diffusion are reviewed, including the dependence of penetration distance from vessels into tissue on solute binding or uptake in tissue. The effects of the abnormal characteristics of tumour vasculature and extravascular tissue on these transport properties are then discussed. Finally, methods for overcoming limitations in drug transport and thereby achieving improved therapeutic results are surveyed.
Conflict of interest statement
Competing interests statement
T.W.S. has no conflicts of interest. M.W.D. was involved in the development of the thermally sensitive liposome described in this paper and has stock in Celsion Corporation, the company that licensed the drug. M.W.D. is also a consultant for Siva Therapeutics and Kaio Therapy and a member of the Scientific Advisory Board of Innovate Biopharmaceuticals.
Figures
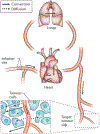
Figure 1 ∣. The pathway that drugs take from an intravenous infusion site to a solid tumour.
Drug is transported by convection in the bloodstream through the systemic veins, the heart, the lungs and the systemic arteries to peripheral microvessels. Owing to rapid mixing of solutes in the blood, all parts of the circulatory system are exposed to the drug. Exchange between blood and tissue occurs primarily inthe microcirculation. Drug passes through microvessel walls and extravascular tissues to cancer cells by a combination of molecular diffusion and convection in interstitial fluid. Convective fluid motion in tissue is driven by fluid that filters through vessel walls. This fluid loss is balanced by resorption through other vessels and by the lymphatic system. For low-relative-molecular-mass drugs, diffusion is dominant over convection in the extravascular space.
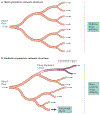
Figure 2 ∣. Limitations of drug transport within the microcirculation.
a ∣ Microvascular networks are often depicted as having ideal symmetric branching structures so that drugs in the blood are uniformly distributed throughout the branches of the network, b ∣ In reality, microvascular networks have highly asymmetric structures with extensive variation among branches in flow path lengths and blood flow rates. Some branches receive very low flow, and the drug may be depleted before the terminal branches are reached, such that some tissue regions are not treated. Conversely, short high-flow pathways may form functional shunts between the arterial and venous systems, greatly restricting exchange of solute between blood and tissue. This effect is accentuated in tumours relative to normal tissues.
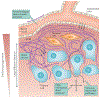
Figure 3 ∣. Limitations of drug transport in extravascular tissue.
A drug must pass several potential barriers in order to reach tumour cells. High tissue pressures may cause vessel collapse, restricting blood flow. The endothelial cells lining microvessels restrict extravasation of drug. Dense stroma, consisting of extracellular matrix (ECM) and cells such as fibroblasts, can be a physical barrier, particularly for large molecules and nanoparticles, and is a binding site for some drugs. The pathway for transport within tumours may be tortuous owing to stroma and parenchymal cells. Large transport distances may result in incomplete drug distribution. High interstitial fluid pressure (IFP) acting in all directions (denoted by the crossed arrows) may reduce fluid flow and restrict drug delivery by convection. Pink background shading represents the oxygen level decreasing with distance from the blood vessel.
Figure 4 ∣. Principle of thermally triggered drug release from liposomes.
This illustration is based on experimental imaging of drug distribution. Local application of heat during the delivery of liposomes causes pores to form in the lipid bilayer and thus release of drug, which diffuses into the tissue. With heating, penetration of liposomes through blood vessel walls into tissue is not required for drug delivery to tumour. Here, drug release occurs intravascularly. Green shading indicates the level of drug.
Similar articles
- Computational modelling of drug delivery to solid tumour: Understanding the interplay between chemotherapeutics and biological system for optimised delivery systems.
Zhan W, Alamer M, Xu XY. Zhan W, et al. Adv Drug Deliv Rev. 2018 Jul;132:81-103. doi: 10.1016/j.addr.2018.07.013. Epub 2018 Jul 29. Adv Drug Deliv Rev. 2018. PMID: 30059703 Review. - Determinants of drug delivery and transport to solid tumors.
Au JL, Jang SH, Zheng J, Chen CT, Song S, Hu L, Wientjes MG. Au JL, et al. J Control Release. 2001 Jul 6;74(1-3):31-46. doi: 10.1016/s0168-3659(01)00308-x. J Control Release. 2001. PMID: 11489481 Review. - Drug delivery and transport to solid tumors.
Jang SH, Wientjes MG, Lu D, Au JL. Jang SH, et al. Pharm Res. 2003 Sep;20(9):1337-50. doi: 10.1023/a:1025785505977. Pharm Res. 2003. PMID: 14567626 Review. - Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies.
Chauhan VP, Stylianopoulos T, Boucher Y, Jain RK. Chauhan VP, et al. Annu Rev Chem Biomol Eng. 2011;2:281-98. doi: 10.1146/annurev-chembioeng-061010-114300. Annu Rev Chem Biomol Eng. 2011. PMID: 22432620 Review. - In-silico dynamic analysis of cytotoxic drug administration to solid tumours: Effect of binding affinity and vessel permeability.
Vavourakis V, Stylianopoulos T, Wijeratne PA. Vavourakis V, et al. PLoS Comput Biol. 2018 Oct 8;14(10):e1006460. doi: 10.1371/journal.pcbi.1006460. eCollection 2018 Oct. PLoS Comput Biol. 2018. PMID: 30296260 Free PMC article.
Cited by
- Multi-Scale and Multi-Physics Models of the Transport of Therapeutic/Diagnostic Cancer Agents.
Moradi Kashkooli F, Kolios MC. Moradi Kashkooli F, et al. Cancers (Basel). 2023 Dec 15;15(24):5850. doi: 10.3390/cancers15245850. Cancers (Basel). 2023. PMID: 38136395 Free PMC article. - Effect of Collagen Matrix on Doxorubicin Distribution and Cancer Cells' Response to Treatment in 3D Tumor Model.
Druzhkova I, Nikonova E, Ignatova N, Koryakina I, Zyuzin M, Mozherov A, Kozlov D, Krylov D, Kuznetsova D, Lisitsa U, Shcheslavskiy V, Shirshin EA, Zagaynova E, Shirmanova M. Druzhkova I, et al. Cancers (Basel). 2022 Nov 8;14(22):5487. doi: 10.3390/cancers14225487. Cancers (Basel). 2022. PMID: 36428580 Free PMC article. - Biodegradable manganese-doped hydroxyapatite antitumor adjuvant as a promising photo-therapeutic for cancer treatment.
Park S, Choi J, Doan VHM, O SH. Park S, et al. Front Mol Biosci. 2022 Nov 23;9:1085458. doi: 10.3389/fmolb.2022.1085458. eCollection 2022. Front Mol Biosci. 2022. PMID: 36504716 Free PMC article. - A Theranostic Nanocomplex Combining with Magnetic Hyperthermia for Enhanced Accumulation and Efficacy of pH-Triggering Polymeric Cisplatin(IV) Prodrugs.
Qu Y, Wang Z, Sun M, Zhao T, Zhu X, Deng X, Zhang M, Xu Y, Liu H. Qu Y, et al. Pharmaceuticals (Basel). 2022 Apr 14;15(4):480. doi: 10.3390/ph15040480. Pharmaceuticals (Basel). 2022. PMID: 35455477 Free PMC article. - Rationale for hypoxia assessment and amelioration for precision therapy and immunotherapy studies.
Dewhirst MW, Mowery YM, Mitchell JB, Cherukuri MK, Secomb TW. Dewhirst MW, et al. J Clin Invest. 2019 Feb 1;129(2):489-491. doi: 10.1172/JCI126044. Epub 2019 Jan 7. J Clin Invest. 2019. PMID: 30614815 Free PMC article. Review. No abstract available.
References
- Bast RC et al. Holland-Frei Cancer Medicine 9th edn Wiley-Blackwell, 2017).
- Swabb EA, Wei J & Gullino PM Diffusion and convection in normal and neoplastic tissues. Cancer Res 34, 2814–2822 (1974). - PubMed
- In this early account of how tissue glycosaminoglycan content can affect solute transport by convection compared with by diffusion in tumours, the authors suggest that degradation of glycosaminoglycan with hyaluronidase could improve solute transport in tumours.
- Jain RK Transport of molecules across tumor vasculature. Cancer Metastasis Rev 6, 559–593 (1987). - PubMed
- This comprehensive review discusses the transport properties of microvessel walls with respect to their importance for drugs of various sizes.
- Jain RK Transport of molecules in the tumor interstitium: a review. Cancer Res 47, 3039–3051 (1987). - PubMed
- This review focuses on factors that impede solute transport in the interstitium of tumours and shows how high IFP and lack of functioning lymphatics contribute to reduced transport.
- Jain RK The Eugene M. Landis Award Lecture 1996. Delivery of molecular and cellular medicine to solid tumors. Microcirculation 4, 1–23 (1997). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources