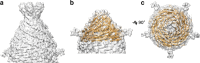Unique architecture of thermophilic archaeal virus APBV1 and its genome packaging - PubMed (original) (raw)
Unique architecture of thermophilic archaeal virus APBV1 and its genome packaging
Denis Ptchelkine et al. Nat Commun. 2017.
Abstract
Archaeal viruses have evolved to infect hosts often thriving in extreme conditions such as high temperatures. However, there is a paucity of information on archaeal virion structures, genome packaging, and determinants of temperature resistance. The rod-shaped virus APBV1 (Aeropyrum pernix bacilliform virus 1) is among the most thermostable viruses known; it infects a hyperthermophile Aeropyrum pernix, which grows optimally at 90 °C. Here we report the structure of APBV1, determined by cryo-electron microscopy at near-atomic resolution. Tight packing of the major virion glycoprotein (VP1) is ensured by extended hydrophobic interfaces, and likely contributes to the extreme thermostability of the helical capsid. The double-stranded DNA is tightly packed in the capsid as a left-handed superhelix and held in place by the interactions with positively charged residues of VP1. The assembly is closed by specific capping structures at either end, which we propose to play a role in DNA packing and delivery.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Fig. 1
Cryo-EM and 3D reconstruction of APBV1. a Cryo-electron micrograph showing APBV1 viral particles in vitrous ice. Scale bar 10 nm. b 2D class average of the whole APBV1 particle. Scale bar 10 nm. c Three-dimensional model of complete APBV1 particle is shown from the side. Scale bar 10 nm. d, e Top (d) and side (e) view of APBV1 map (transparent surface) and model of the major capsid protein VP1. VP1 subunits related by C5 symmetry are colored in red, yellow, green, orange, and magenta
Fig. 2
Interactions within and between VP1 subunits. a The cryo-EM density (mesh) and corresponding model (sticks) of VP1 are shown. In addition to the positively charged residues at the N (Lys9) and C (Arg79) termini and the putative glycosylation site at Asn47, key residues involved in intramolecular interactions are labeled. b Two VP1 molecules are rendered as a ribbon in orange and blue. Residues involved in intermolecular contacts between two neighboring VP1 subunits are labeled. These residues create multiple hydrophobic contacts and a large interface
Fig. 3
APBV1 DNA adopts the left-handed superhelix form. a Electrostatic surface of APBV1 is shown from inside the virion. DNA follows the positively charged tracks made up by the residues Lys9 and Arg79 of the VP1 protein. b Model of APBV1 DNA shows a left-handed superhelical organization. The DNA passing through the middle of the DNA superhelix was not modeled
Fig. 4
Reconstruction of the APBV1 cap structures. a EM map of “pointy” tip and end fibers possibly involved in recognition of the host receptor proteins. b, c EM map of “blunt” tip is shown from the side (b) and the top (c). The tip was modeled by fitting VP1 structure as a rigid body. The cap structure has five-fold symmetry. The tilt of the subunits changes with each level on approach to the tip of the cap. The additional densities on the sides of the cap were putatively assigned to a glycan attached to the VP1 subunit residue Asn47
Similar articles
- Diversity of viruses of the hyperthermophilic archaeal genus Aeropyrum, and isolation of the Aeropyrum pernix bacilliform virus 1, APBV1, the first representative of the family Clavaviridae.
Mochizuki T, Yoshida T, Tanaka R, Forterre P, Sako Y, Prangishvili D. Mochizuki T, et al. Virology. 2010 Jul 5;402(2):347-54. doi: 10.1016/j.virol.2010.03.046. Epub 2010 Apr 28. Virology. 2010. PMID: 20430412 - Archaeal virus with exceptional virion architecture and the largest single-stranded DNA genome.
Mochizuki T, Krupovic M, Pehau-Arnaudet G, Sako Y, Forterre P, Prangishvili D. Mochizuki T, et al. Proc Natl Acad Sci U S A. 2012 Aug 14;109(33):13386-91. doi: 10.1073/pnas.1203668109. Epub 2012 Jul 23. Proc Natl Acad Sci U S A. 2012. PMID: 22826255 Free PMC article. - Provirus induction in hyperthermophilic archaea: characterization of Aeropyrum pernix spindle-shaped virus 1 and Aeropyrum pernix ovoid virus 1.
Mochizuki T, Sako Y, Prangishvili D. Mochizuki T, et al. J Bacteriol. 2011 Oct;193(19):5412-9. doi: 10.1128/JB.05101-11. Epub 2011 Jul 22. J Bacteriol. 2011. PMID: 21784945 Free PMC article. - Structure and assembly of archaeal viruses.
Baquero DP, Liu Y, Wang F, Egelman EH, Prangishvili D, Krupovic M. Baquero DP, et al. Adv Virus Res. 2020;108:127-164. doi: 10.1016/bs.aivir.2020.09.004. Epub 2020 Oct 5. Adv Virus Res. 2020. PMID: 33837715 Review. - Survey of high-resolution archaeal virus structures.
Hartman R, Munson-McGee J, Young MJ, Lawrence CM. Hartman R, et al. Curr Opin Virol. 2019 Jun;36:74-83. doi: 10.1016/j.coviro.2019.05.008. Epub 2019 Jun 22. Curr Opin Virol. 2019. PMID: 31238245 Review.
Cited by
- Self-assembly of Aeropyrum pernix bacilliform virus 1 (APBV1) major capsid protein and its application as building blocks for nanomaterials.
Sumikama Y, Takashima A, Mochizuki T, Sakuraba H, Ohshima T, Sugihara S, Suye SI, Satomura T. Sumikama Y, et al. Extremophiles. 2022 Nov 13;26(3):34. doi: 10.1007/s00792-022-01284-x. Extremophiles. 2022. PMID: 36372831 - Principles for enhancing virus capsid capacity and stability from a thermophilic virus capsid structure.
Stone NP, Demo G, Agnello E, Kelch BA. Stone NP, et al. Nat Commun. 2019 Oct 2;10(1):4471. doi: 10.1038/s41467-019-12341-z. Nat Commun. 2019. PMID: 31578335 Free PMC article. - New virus isolates from Italian hydrothermal environments underscore the biogeographic pattern in archaeal virus communities.
Baquero DP, Contursi P, Piochi M, Bartolucci S, Liu Y, Cvirkaite-Krupovic V, Prangishvili D, Krupovic M. Baquero DP, et al. ISME J. 2020 Jul;14(7):1821-1833. doi: 10.1038/s41396-020-0653-z. Epub 2020 Apr 22. ISME J. 2020. PMID: 32322010 Free PMC article. - Archaeal Viruses from High-Temperature Environments.
Munson-McGee JH, Snyder JC, Young MJ. Munson-McGee JH, et al. Genes (Basel). 2018 Feb 27;9(3):128. doi: 10.3390/genes9030128. Genes (Basel). 2018. PMID: 29495485 Free PMC article. Review. - Structure of a filamentous virus uncovers familial ties within the archaeal virosphere.
Wang F, Baquero DP, Su Z, Osinski T, Prangishvili D, Egelman EH, Krupovic M. Wang F, et al. Virus Evol. 2020 Apr 29;6(1):veaa023. doi: 10.1093/ve/veaa023. eCollection 2020 Jan. Virus Evol. 2020. PMID: 32368353 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 093305/Z/10/Z/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MC_EX_G0901251/MRC_/Medical Research Council/United Kingdom
- 203141/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 060208/Z/00/Z/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources