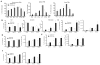Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors - PubMed (original) (raw)
. 2017 Nov 13;32(5):654-668.e5.
doi: 10.1016/j.ccell.2017.10.005.
Laxminarasimha Donthireddy 1, Douglas Marvel 1, Thomas Condamine 1, Fang Wang 1, Sergio Lavilla-Alonso 1, Ayumi Hashimoto 1, Prashanthi Vonteddu 1, Reeti Behera 1, Marlee A Goins 2, Charles Mulligan 2, Brian Nam 2, Neil Hockstein 2, Fred Denstman 2, Shanti Shakamuri 2, David W Speicher 3, Ashani T Weeraratna 1, Timothy Chao 4, Robert H Vonderheide 4, Lucia R Languino 5, Peter Ordentlich 6, Qin Liu 1, Xiaowei Xu 4, Albert Lo 7, Ellen Puré 7, Chunsheng Zhang 8, Andrey Loboda 8, Manuel A Sepulveda 9, Linda A Snyder 9, Dmitry I Gabrilovich 10
Affiliations
- PMID: 29136508
- PMCID: PMC5827952
- DOI: 10.1016/j.ccell.2017.10.005
Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors
Vinit Kumar et al. Cancer Cell. 2017.
Abstract
Tumor-associated macrophages (TAM) contribute to all aspects of tumor progression. Use of CSF1R inhibitors to target TAM is therapeutically appealing, but has had very limited anti-tumor effects. Here, we have identified the mechanism that limited the effect of CSF1R targeted therapy. We demonstrated that carcinoma-associated fibroblasts (CAF) are major sources of chemokines that recruit granulocytes to tumors. CSF1 produced by tumor cells caused HDAC2-mediated downregulation of granulocyte-specific chemokine expression in CAF, which limited migration of these cells to tumors. Treatment with CSF1R inhibitors disrupted this crosstalk and triggered a profound increase in granulocyte recruitment to tumors. Combining CSF1R inhibitor with a CXCR2 antagonist blocked granulocyte infiltration of tumors and showed strong anti-tumor effects.
Keywords: CSF1R; M-CSF; PMN-MDSC; fibroblasts; granulocytes; macrophages.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
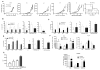
Figure 1. Effect of CSF1R inhibitor on tumor infiltration by PMN-MDSC
A. Effect of JNJ-40346527 treatment on tumor growth in different tumor models. JNJ-40346527 was administered 6 days a week orally (20 mg/kg) from one day after tumor inoculation and continued until end of observation. Tumors size in mm2 is shown. Each group included 5–9 mice. Means and SD are shown. B–D. The proportion of cells in tumors from mice at the end of the treatment as described in Fig. 1A. Each group included 5–8 mice. Means and SD are shown. * - p<0.05, **- p<0.01, *** - p<0.001 in Student’s t-test from vehicle control group. B. CD11b+Gr-1−F4/80hi TAM. C. CD11b+Ly6ChiLy6G− monocytic cells. D. CD11b+Ly6CloLy6G+ granulocytic cells. E. Immune suppressive activity of Ly6G+ cells isolated from tumors of JNJ-40346527 treated mice. Ly6G+ cells were added at indicated ratios to splenocytes from Pmel transgenic mice and stimulated with cognate peptide. Proliferation was measured by 3H-thymidine incorporation in triplicates. Two experiments with the same results were performed. **- p<0.01, *** - p<0.001 in Student’s t-test from splenocytes alone. F. The absolute number of myeloid cells per gram tumor of JNJ-40346527 treated mice in indicated tumor models. See also Figures S1.
Figure 2. CSF1R inhibitor causes recruitment of PMN-MDSC to tumors
A. The proportion of CD11b+Ly6CloLy6G+ PMN-MDSC in different tissues of mice treated with JNJ-40346527 at the end of the treatment. Each group included 3–4 mice. * - p<0.05 in Student’s t-test from vehicle only group. B. Proportion of myeloid cells in tumors evaluated at different time during the treatment of LLC-TB mice with JNJ-40346527. Each group included 4 mice. Mean and SD are shown. * - p<0.05 from vehicle control group. C–D. The proportion and absolute number of PMN-MDSC of LLC-TB mice treated with JNJ-40346527. in spleen (C) in BM (D) E–F Proportion and absolute number of M-MDSC of LLC-TB mice treated with JNJ-40346527 in spleen (E) in BM (F) Mean and SD are shown (n=4). * - p<0.05 from vehicle control group. See also Figures S2 and S3.
Figure 3. Granulocytic chemokines in tumors of CSF1R inhibitor treated mice
A and B. Expression of mRNA (by qPCR) of indicated chemokines in tumor tissues treated with JNJ-40346527 for 16 days or 2 weeks respectively. LLC-TB mice (A) TRAMP mice (B) Mean and SD (n=3) are shown. * - p<0.05 in Student’s t-test from vehicle control group. C. Amount of Cxcl1 (measured in lysates by ELISA) in tumors of mice treated with JNJ-40346527 for 9, 16 or 23 days as described. Mean and SD (n=4) are shown * - p<0.05; ** - p<0.01 in Student’s t-test from vehicle control group. D. Endogenous level of Cxcl1 measured in lysates by ELISA in different cells isolated from tumors of LLC-TB mice. Fibroblasts – PDGFRa+ cells, tumor cells – CD45-EPCAM+, myeloid cells – CD45+CD11b+. Mean and SD are shown. * p<0.05; *** p<0.001 in in Student’s t-test from fibroblasts values. E. Cxcl1 in different cells isolated from tumors of LLC-TB mice treated with JNJ-40346527 for 16 days. ** - p<0.01 in Student’s t-test from vehicle control group (n=3). F. Cxcl-1 in fibroblasts isolated from tumors of LLC-TB mice treated with CD115 antibody twice a week for 3 weeks. *** - p<0.001 in Student’s t-test from vehicle control group (n=3). G. Cxcl1 expression by qPCR in pancreatic ductal adenocarcinoma treated with FAP specific CAR T-cells. Tumors were collected 3 days after the injection of second dose of CAR T-cells. * - p<0.05 from control (n=4). H. Ly6G+ cells in tumor tissues of mice treated with FAP CAR-T cells with atypical staining (red – Ly6G, blue-DAPI), scale bar = 50 μm and quantitative results shown (n=4). ** - p<0.05 in Student’s t-test from control. I. Ly6G+ cells per mm2 in tumor tissues of mice treated with FAP CAR-T cells. Tumors were collected 3 days after CAR-T cell injection. * - p<0.05 in Student’s t-test from control (n=4). J. Cxcl1 expression (by qPCR) in AE17.ova mesothelioma tumors from mice treated with FAP specific CAR-T cells. * - p<0.05 from control.
Figure 4. Effect of CSF1 on fibroblasts’ ability to recruit PMN-MDSC
A. Expression of CD115 on the surface of fibroblasts tested by FACs and in cell lysates of cultured CAF tested by western blot B. Expression of Cxcl1 mRNA (qPCR) (left) and Cxcl-1 protein (ELISA) (right) in normal lung fibroblasts treated with Csf1 with and without JNJ-40346527. Mean and SD are shown (n=3). **- p<0.01, *** - p<0.001 in Student’s t-test from control group. C. Effect of Csf1 on fibroblast-mediated recruitment of BM PMN-MDSC. Chemotaxis of BM PMN-MDSC from LLC-TB mice was evaluated against supernatants obtained from fibroblasts cultured with Csf1 and Csf1R inhibitor. *- p<0.05 in Student’s t-test from control group (n=4). D. PMN-MDSC chemotaxis to supernatants from fibroblasts in presence of neutralizing Csf1 antibody (Ab). *- p<0.05 from control group in Student’s t-test (n=3). E. PMN-MDSC chemotaxis to supernatants from fibroblasts in presence of neutralizing CXCL1 Ab. **-p<0.01 from control group in Student’s t-test. F. PMN-MDSC chemotaxis to recombinant CXCL1 (100 ng/ml) in the presence of Csf1 and CSF1R inhibitor ***- p<0.001 in Student’s t-test from control group (n=3). G. Csf1 ELISA with TES collected from control or JNJ-40346527-treated LLC TB mice. *- p<0.05 in Student’s t-test from control group (n=4). H. PMN-MDSC chemotaxis in presence of TES collected from control or JNJ-40346527-treated LLC TB mice. *- p<0.05 in Student’s t-test from control group (n=4). I. LLC tumor growth kinetics in WT and CXCR2 KO mice with or without JNJ-40346527 treatment for indicated days. J. The absolute counts of PMN-MDSC and TAM in tumors of WT or CXCR2 KO TB mice treated with JNJ-40346527. *- p<0.05 in Student’s t-test from control group (n=4). See also Figure S4.
Figure 5. Effect of CSF1 on fibroblasts
A. Presence of myeloid cells in LLC tumors with deleted CSF1. WT and CSF1 deleted tumors were collected from mice 4–5 weeks after inoculation. Mean and SD are shown (n=4). *- p<0.05 from WT tumors. B. Expression (Means and SD) of chemokines mRNA (by qPCR) in these tumors. *- p<0.05, ***-p<0.001 in Student’s t-test from respective controls. C. PMN-MDSC chemotaxis in the presence of fibroblasts treated with supernatants from WT or CSF1 KO LLC. **-p<0.01 in Student’s t-test from control group (n=3). D. Supernatants were collected from fibroblasts with silenced csf1r expression (CSF1R shRNA) treated for 4 days with CSF1. Supernatants were used in chemotaxis of BM PMN-MDSC (n=3). *** - p<0.001 in Student’s t-test from control. E. Fibroblasts transduced with control or Csf1r shRNA were mixed at 1:1 ratio with LLC tumor cells and injected s.c. to mice. Twelve days later, the presence of myeloid cells in tumors was evaluated (n=4). Mean and SD are shown ** - p<0.01 in Student’s t-test from control. F. Expression of CSF1R (CD115) on cells in tumor microenvironment (CD3 T cells, FAP fibroblasts, CD163 macrophages) from 4 different patients. GeoMean is shown. G. Fibroblasts isolated from resected lung tissues of patients with NSCLC were treated for 4 days with CSF1 followed by JNJ-40346527 for additional 2 days. Expression of CXCL8 by qPCR (n=3) Mean and SD are shown. ** - p<0.01 in Student’s t-test from control. H. Migration of peripheral blood donor’s neutrophils to supernatants from fibroblasts cultured with CSF1 and CSF1R inhibitor (n=3), * - p<0.05 from control. Means and SD are shown. I. Peripheral blood donor’s neutrophils chemotaxis to supernatants from fibroblasts in presence of CXCL8 neutralizing antibody (n=3). * - p<0.05 in Student’s t-test from control. Means and SD are shown. See also Figures S4 and S5
Figure 6. Association between CSF1 and PMN-MDSC recruitment to human tumors
A. Effect of CSF1R expression silencing in human fibroblasts on their ability to induce neutrophil migration. CSF1R was silenced in human fibroblasts using lentiviral shRNA construct and then cells were treated with CSF1 for 5 days. Supernatant was used in chemotaxis of donor peripheral blood neutrophils. Control – fibroblasts infected with lentivirus containing empty vector or the construct which did not down-regulate CSF1R. (n=3), ** - p<0.01 in Student’s t-test from control. Mean and SD are shown. B. Migration of peripheral blood donor’s neutrophils to recombinant CXCL8 (100 ng/ml) in the presence CSF1 and CSF1R inhibitor. Mean and SD are shown. n = 3. *- p<0.05 from control group. C–G. Evaluation of the proportion of tumor PMN-MDSC and M-MDSC and amount of cytokines in tumors of cancer patients. Values of individual tumor samples are plotted. The proportion of intratumoral CD11b+CD14−CD15+ PMN-MDSC among CD45+ cells evaluated by flow cytometry. CXCL8 in tumor cell lysates was measured by ELISA and split based on median (7.84 pg/μg) (C). CD14+HLA-DR−/lo M-MDSC among CD45+ cells (D). The proportion of PMN-MDSC in tumor samples split based on the amount of CSF1 present (median 7.5 pg/μg) (E). M-MDSC in samples split based on CSF1 amount in tumors (F). Amount of CXCL8 in tumor samples split based on CSF1 median (G). Mean and SD are shown. p values in Student’s t-test are shown on the graphs. H. Correlation between the amount of CSF1 and CXCL8 proteins in secretomes from 15 ovarian tumors (top panel). Spearman correlation coefficient was calculated. Analysis of the same samples based on median of CSF1 amount (bottom panel). Mean and SD are shown. p values in Student’s t-test I. Survival of patients with HNC based on the number of PMN-MDSC (CD15+LOX-1+) in tumor tissues. TMA contained samples from 58 patients with pathologically confirmed HNC. Median of PMN-MDSC presence per mm2 in all samples was 50. Survival log rank test was used. J. The number of PMN-MDSC in tumor tissues (per mm2) in TMA samples divided based on median score of M-CSF staining (160). K. Correlation between neutrophil and monocyte gene signatures in tumor samples (19,000 samples from Merck & Co., Inc., Kenilworth, NJ USA database) and expression of CSF1 and CSF2. **** - p<0.00001 calculated between indicated pairs. Mann-Whitney U test was used. The box-and-whisker plots are show. The box shows the median as well as 25th and 75th percentile. The whiskers stretch to the maximum and minimum values of that variable. See also Figure S6.
Figure 7. The mechanisms regulating inhibition of chemokines production by CSF1
A. mRNA for Cxcl1 (by qPCR) (left) and Cxcl1 protein by ELISA (right) in mouse fibroblasts cultured for 6 days with CSF1 in the presence (during last three days of culture) of class I HDAC inhibitor entinostat. Mean and SD (n=3) are shown. ***-p<0.001 in Student’s t test from control. B–F. The effect of entinostat in vivo. LLC TB mice were treated with entinostat (10 mg/kg, p.o.) daily for 10 days starting from day 15 after tumor inoculation. Expression of mRNA for chemokines (qPCR) in tumor tissues. **-p<0.01 in Student’s t test from control (n=3) (B). Tumor cells were isolated at the end of the treatment and the amount of CSF1 was measured by ELISA (n=6) (C). CAF were sorted from tumor tissues and the amount of Cxcl1 was measured in cell lysates by ELISA. Mean and SD are shown. ****-p<0.0001 in Student’s t test from control (n=4) (D). CSF1R amount in CAF (E). The presence of PMN-MDSC and TAM in tumors of LLC (n=10) and CT-26 (n=3) TB mice treated with entinostat. Mean and SD are shown *-p<0.05; **-p<0.01 in Student’s t test from control (F). G. Mouse fibroblasts were treated with CSF1 for 4 days, followed by 2-day treatment of entinostat. ChIP of Cxcl1 promoter was performed using acetylated histone H3 or acetylated histone H4 antibody. The results are expressed as DNA enrichment, normalized to corresponding input values. Control IgG was used as a negative control (n=3). H. ChIP of Cxcl1 promoter was performed using HDAC2 specific antibody (n=3).
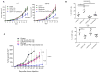
Figure 8. Therapeutic targeting of granulocytes and macrophages
A. Tumor growth in mice treated with JNJ-40346527 (CSF1R inhibitor), SB225002 (CXCR2 inhibitor), or their combination. Mean and SD are shown. Each group included 5 mice. Significance was calculated using two-way ANOVA test. B. The presence of TAM and PMN-MDSC in B16F10 tumors. **-p<0.01 from control (n=4). C. LLC TB mice were treated with inhibitors and PD-1 antibody (100 μg twice a week). Mean and SD are shown. Each group included 4–5 mice. Significance was calculated using a linear mixed-effect model with the random effect at individual animal level.
Comment in
- Does CSF1R Blockade Turn into Friendly Fire?
Greten TF. Greten TF. Cancer Cell. 2017 Nov 13;32(5):546-547. doi: 10.1016/j.ccell.2017.10.012. Cancer Cell. 2017. PMID: 29136500 Free PMC article.
Similar articles
- Myeloid-Derived Suppressor Cell Subset Accumulation in Renal Cell Carcinoma Parenchyma Is Associated with Intratumoral Expression of IL1β, IL8, CXCL5, and Mip-1α.
Najjar YG, Rayman P, Jia X, Pavicic PG Jr, Rini BI, Tannenbaum C, Ko J, Haywood S, Cohen P, Hamilton T, Diaz-Montero CM, Finke J. Najjar YG, et al. Clin Cancer Res. 2017 May 1;23(9):2346-2355. doi: 10.1158/1078-0432.CCR-15-1823. Epub 2016 Oct 31. Clin Cancer Res. 2017. PMID: 27799249 Free PMC article. - Pancreatic Ductal Adenocarcinoma (PDAC) circulating tumor cells influence myeloid cell differentiation to support their survival and immunoresistance in portal vein circulation.
Arnoletti JP, Reza J, Rosales A, Monreal A, Fanaian N, Whisner S, Srivastava M, Rivera-Otero J, Yu G, Phanstiel Iv O, Altomare DA, Tran Q, Litherland SA. Arnoletti JP, et al. PLoS One. 2022 Mar 22;17(3):e0265725. doi: 10.1371/journal.pone.0265725. eCollection 2022. PLoS One. 2022. PMID: 35316296 Free PMC article. - CSF1R signaling blockade stanches tumor-infiltrating myeloid cells and improves the efficacy of radiotherapy in prostate cancer.
Xu J, Escamilla J, Mok S, David J, Priceman S, West B, Bollag G, McBride W, Wu L. Xu J, et al. Cancer Res. 2013 May 1;73(9):2782-94. doi: 10.1158/0008-5472.CAN-12-3981. Epub 2013 Feb 15. Cancer Res. 2013. PMID: 23418320 Free PMC article. - Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy.
Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Rüttinger D. Cannarile MA, et al. J Immunother Cancer. 2017 Jul 18;5(1):53. doi: 10.1186/s40425-017-0257-y. J Immunother Cancer. 2017. PMID: 28716061 Free PMC article. Review. - The M-CSF receptor in osteoclasts and beyond.
Mun SH, Park PSU, Park-Min KH. Mun SH, et al. Exp Mol Med. 2020 Aug;52(8):1239-1254. doi: 10.1038/s12276-020-0484-z. Epub 2020 Aug 17. Exp Mol Med. 2020. PMID: 32801364 Free PMC article. Review.
Cited by
- Targeting the MET gene: unveiling therapeutic opportunities in immunotherapy within the tumor immune microenvironment of non-small cell lung cancer.
Ye L, Wang W, Li H, Ji Y, Le X, Xu X. Ye L, et al. Ther Adv Med Oncol. 2024 Oct 17;16:17588359241290733. doi: 10.1177/17588359241290733. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39483139 Free PMC article. Review. - Insights into CSF-1R Expression in the Tumor Microenvironment.
Tomassetti C, Insinga G, Gimigliano F, Morrione A, Giordano A, Giurisato E. Tomassetti C, et al. Biomedicines. 2024 Oct 18;12(10):2381. doi: 10.3390/biomedicines12102381. Biomedicines. 2024. PMID: 39457693 Free PMC article. Review. - Macrophages: Key Players in the Battle against Triple-Negative Breast Cancer.
Padzińska-Pruszyńska I, Kucharzewska P, Matejuk A, Górczak M, Kubiak M, Taciak B, Król M. Padzińska-Pruszyńska I, et al. Int J Mol Sci. 2024 Oct 7;25(19):10781. doi: 10.3390/ijms251910781. Int J Mol Sci. 2024. PMID: 39409110 Free PMC article. Review. - Mechanistic studies of tumor-associated macrophage immunotherapy.
Cao J, Liu C. Cao J, et al. Front Immunol. 2024 Sep 30;15:1476565. doi: 10.3389/fimmu.2024.1476565. eCollection 2024. Front Immunol. 2024. PMID: 39403370 Free PMC article. Review. - Consensus, debate, and prospective on pancreatic cancer treatments.
Wang J, Yang J, Narang A, He J, Wolfgang C, Li K, Zheng L. Wang J, et al. J Hematol Oncol. 2024 Oct 10;17(1):92. doi: 10.1186/s13045-024-01613-x. J Hematol Oncol. 2024. PMID: 39390609 Free PMC article. Review.
References
- Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, Cloughesy TF, Marimuthu A, Haidar S, Perry A, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2016;18:557–564. - PMC - PubMed
- Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;81:130–137. - PubMed
- Challacombe JM, Suhrbier A, Parsons PG, Jones B, Hampson P, Kavanagh D, Rainger GE, Morris M, Lord JM, Le TT, et al. Neutrophils are a key component of the antitumor efficacy of topical chemotherapy with ingenol-3-angelate. J Immunol. 2006;177:8123–8132. - PubMed
- Condamine T, Dominguez G, Youn J, Kossenkov A, Mony S, Alicea-Torres K, Tcyganov E, Hashimoto A, Nefedova Y, Lin C, et al. Lectin-type oxidized LDL receptor 1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Science Immunol. 2016:1. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 CA140043/CA/NCI NIH HHS/United States
- P30 CA010815/CA/NCI NIH HHS/United States
- P01 CA114046/CA/NCI NIH HHS/United States
- R01 CA084488/CA/NCI NIH HHS/United States
- R01 CA174746/CA/NCI NIH HHS/United States
- P50 CA168536/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous