Chromatin accessibility dynamics reveal novel functional enhancers in C. elegans - PubMed (original) (raw)
Chromatin accessibility dynamics reveal novel functional enhancers in C. elegans
Aaron C Daugherty et al. Genome Res. 2017 Dec.
Abstract
Chromatin accessibility, a crucial component of genome regulation, has primarily been studied in homogeneous and simple systems, such as isolated cell populations or early-development models. Whether chromatin accessibility can be assessed in complex, dynamic systems in vivo with high sensitivity remains largely unexplored. In this study, we use ATAC-seq to identify chromatin accessibility changes in a whole animal, the model organism Caenorhabditis elegans, from embryogenesis to adulthood. Chromatin accessibility changes between developmental stages are highly reproducible, recapitulate histone modification changes, and reveal key regulatory aspects of the epigenomic landscape throughout organismal development. We find that over 5000 distal noncoding regions exhibit dynamic changes in chromatin accessibility between developmental stages and could thereby represent putative enhancers. When tested in vivo, several of these putative enhancers indeed drive novel cell-type- and temporal-specific patterns of expression. Finally, by integrating transcription factor binding motifs in a machine learning framework, we identify EOR-1 as a unique transcription factor that may regulate chromatin dynamics during development. Our study provides a unique resource for C. elegans, a system in which the prevalence and importance of enhancers remains poorly characterized, and demonstrates the power of using whole organism chromatin accessibility to identify novel regulatory regions in complex systems.
© 2017 Daugherty et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
Figure 1.
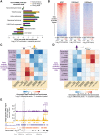
ATAC-seq in whole C. elegans captures chromatin accessibility dynamics across three life-stages. (A) Three independent biological replicates each consisting of tightly temporally synchronized C. elegans were used for ATAC-seq. Hours post-egg lay are at 20°C. (B) C. elegans were flash frozen and nuclei were isolated before assaying accessible chromatin using transposons loaded with next-generation sequencing adaptors, allowing paired-end sequencing. A custom analysis pipeline emphasizing high-resolution signal and consistent peaks, as well as accommodating input control, was developed to generate stage-specific and consensus (i.e., across stages) ATAC-seq peaks. (C) ATAC-seq signal within consensus ATAC-seq peaks was compared between all samples using Spearman's ρ to cluster samples. Replicate batches are noted as letters following the stage. (D,E) Comparison of ATAC-seq signal (normalized by total sequencing depth) between all three stages at a region that decreases (D) or increases (E) in accessibility during development. (F,G) Genes that lose accessibility between embryo and larval stage 3 (L3) are enriched for early development functions (F), while genes that gain accessibility are enriched for larval-related functions (G); all calculations and genes lists are from GOrilla (Eden et al. 2009), and the number of genes enriched in each term are listed in parentheses.
Figure 2.
ATAC-seq as a single assay describes the epigenome. (A) Enrichment of stage-specific ATAC-seq peaks in ChromHMM-predicted chromatin states relative to values expected by chance; significance derived via 10,000 bootstrapping iterations, and error bars reflect 95% confidence intervals (P ≤ 1 × 10−4, except for early embryo transcribed gene body [P = 0.633]). The decrease in enrichment of ATAC-seq peaks in H3K27me3-repressed regions in L3 compared to young adult and early embryo is likely due to the low sequencing depth of the L3 H3K27me3 ChIP-seq. (B) Larval stage 3 (L3) ATAC-seq signal at 19,899 previously defined transcription start sites (TSS ± 0.5 kb) is correlated with active histone modifications (H3K4me3) and anti-correlated with heterochromatin (H3K9me3) around the TSS. (C,D) Regions that increase in accessibility in ATAC-seq peaks are enriched for transitions from inactive chromatin states to active regulatory states (C), while regions that decrease in accessibility are enriched for transitions from active regulatory states to inactive chromatin states (D). (E) An example of an increase in chromatin accessibility overlapping with a transition from heterochromatin to a predicted active enhancer chromatin state. Multiple TSSs have been noted for kin-1, but only the 5′-most is shown here for ease.
Figure 3.
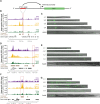
Dynamic ATAC-seq peaks identify functional enhancers with unique spatiotemporal specificity during C. elegans development. (A) Functional enhancer constructs used to generate C. elegans transgenic lines. Putative regulatory regions, or corresponding flanking regions for negative controls, are inserted upstream of a minimal promoter (pes-10) driving green fluorescent protein (GFP) localized to the nucleolus. (B,D,F) Distal (>1 kb from a TSS) noncoding ATAC-seq peaks with the largest fold change in ATAC-seq signal between any two stages were screened for potential enhancer activity. The approximate regions tested near C54G6.2 (B), nhr-25 (D), and swip-10 (F) are boxed in red. Note that for swip-10 (F), the plot orientation is reversed for consistency with other plots. (C,E,G) Specific patterns of spatiotemporal enhancer activity in transgenic lines. Representative images of GFP expression in staged C. elegans transgenic lines are presented with a 50-µm scale bar. All images were straightened with ImageJ and are grayscale images with florescence overlaid.
Figure 4.
Motifs associated with increases in chromatin accessibility during development reveal key transcription factors with unique binding loci. (A) ATAC-seq peaks which decreased (left) or increased (right) accessibility between early embryo and L3 are enriched for previously identified transcription factor binding motifs; _P_-values are Benjamini-Hochberg-corrected for multiple hypothesis testing. (B) The number of instances of previously identified as well as de novo motifs (see
Supplemental Fig. S6C
) in each consensus ATAC-seq peak were used as features in a machine learning model to predict how each ATAC-seq peak changed between early embryo and L3 (increasing, decreasing, or no change). A training set (70% of all ATAC-seq peaks) was used to build the model, while the remaining held-out testing set was used to assess model quality (see
Supplemental Fig. S6D
). (C) The relative influence of every motif from the machine learning model in Figure 4B was quantified. Solid bars are previously defined motifs, while hashed bars are de novo identified motifs in dynamic ATAC-seq peaks. (D) The median L3 ATAC-seq signal and fragment length at the midpoint (±50 bp) of L3 ChIP-seq peaks; box plots of the same data are in
Supplemental Figure S7C,D
. (E) Histograms of L3 ATAC-seq fragment size at the midpoint (±50 bp) of L3 ChIP-seq peaks were calculated and normalized to percentages. Canonical TFs and chromatin factors were then aggregated and plotted. (F) The change in H3 nucleosome occupancy between early embryo and larval stage 3 at the midpoint of each L3 transcription factor ChIP-seq peak was calculated using DANPOS (Chen et al. 2013b) and publicly available H3 ChIP-seq.
Figure 5.
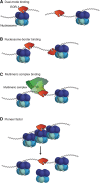
Possible models to explain EOR-1 binding characteristics. EOR-1 could either (A) bind both open and closed chromatin, depending on the genomic loci or the tissues; for example, EOR-1 could bind open sites in one tissue but closed sites in another tissue, (B) bind immediately adjacent to nucleosomes, (C) act as part of a large complex, or (D) act as a pioneer factor by binding and contributing to opening closed chromatin.
Similar articles
- Chromatin accessibility dynamics across C. elegans development and ageing.
Jänes J, Dong Y, Schoof M, Serizay J, Appert A, Cerrato C, Woodbury C, Chen R, Gemma C, Huang N, Kissiov D, Stempor P, Steward A, Zeiser E, Sauer S, Ahringer J. Jänes J, et al. Elife. 2018 Oct 26;7:e37344. doi: 10.7554/eLife.37344. Elife. 2018. PMID: 30362940 Free PMC article. - Genome-wide discovery of active regulatory elements and transcription factor footprints in Caenorhabditis elegans using DNase-seq.
Ho MCW, Quintero-Cadena P, Sternberg PW. Ho MCW, et al. Genome Res. 2017 Dec;27(12):2108-2119. doi: 10.1101/gr.223735.117. Epub 2017 Oct 26. Genome Res. 2017. PMID: 29074739 Free PMC article. - The role of chromatin dynamics in immune cell development.
Winter DR, Amit I. Winter DR, et al. Immunol Rev. 2014 Sep;261(1):9-22. doi: 10.1111/imr.12200. Immunol Rev. 2014. PMID: 25123274 Review. - Roles of chromatin factors in C. elegans development.
Cui M, Han M. Cui M, et al. WormBook. 2007 May 3:1-16. doi: 10.1895/wormbook.1.139.1. WormBook. 2007. PMID: 18050494 Free PMC article. Review.
Cited by
- Whole-genome methods to define DNA and histone accessibility and long-range interactions in chromatin.
Marr LT, Jaya P, Mishra LN, Hayes JJ. Marr LT, et al. Biochem Soc Trans. 2022 Feb 28;50(1):199-212. doi: 10.1042/BST20210959. Biochem Soc Trans. 2022. PMID: 35166326 Free PMC article. Review. - Cellular reprogramming for successful CNS axon regeneration is driven by a temporally changing cast of transcription factors.
Dhara SP, Rau A, Flister MJ, Recka NM, Laiosa MD, Auer PL, Udvadia AJ. Dhara SP, et al. Sci Rep. 2019 Oct 2;9(1):14198. doi: 10.1038/s41598-019-50485-6. Sci Rep. 2019. PMID: 31578350 Free PMC article. - Epigenetics of aging and disease: a brief overview.
Pagiatakis C, Musolino E, Gornati R, Bernardini G, Papait R. Pagiatakis C, et al. Aging Clin Exp Res. 2021 Apr;33(4):737-745. doi: 10.1007/s40520-019-01430-0. Epub 2019 Dec 6. Aging Clin Exp Res. 2021. PMID: 31811572 Free PMC article. Review. - Integrated Chromatin Accessibility and Transcriptome Landscapes of 5-Fluorouracil-Resistant Colon Cancer Cells.
Zhang B, Lin J, Zhang J, Wang X, Deng X. Zhang B, et al. Front Cell Dev Biol. 2022 Feb 17;10:838332. doi: 10.3389/fcell.2022.838332. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35252200 Free PMC article. - Integrative profiling of gene expression and chromatin accessibility elucidates specific transcriptional networks in porcine neutrophils.
Herrera-Uribe J, Lim KS, Byrne KA, Daharsh L, Liu H, Corbett RJ, Marco G, Schroyen M, Koltes JE, Loving CL, Tuggle CK. Herrera-Uribe J, et al. Front Genet. 2023 May 23;14:1107462. doi: 10.3389/fgene.2023.1107462. eCollection 2023. Front Genet. 2023. PMID: 37287538 Free PMC article.
References
- Adkins NL, Hagerman TA, Georgel P. 2006. GAGA protein: a multi-faceted transcription factor. Biochem Cell Biol 84: 559–567. - PubMed
- Altun Z, Hall D. 2009. Muscle system, somatic muscle. In WormAtlas. 10.3908/wormatlas.1.7. - DOI
- Antebi A, Culotti JG, Hedgecock EM. 1998. daf-12 regulates developmental age and the dauer alternative in Caenorhabditis elegans. Development 125: 1191–1205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases