Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy - PubMed (original) (raw)
. 2018 Jan 4;37(1):139-159.
doi: 10.15252/embj.201695709. Epub 2017 Nov 16.
Natalia H Revelo 1, Katharina J Seitz 1 3, Martin S Helm 1 3, Deblina Sarkar 4, Rebecca S Saleeb 5, Elisa D'Este 6, Jessica Eberle 7, Eva Wagner 8 9, Christian Vogl 10 11, Diana F Lazaro 12 13, Frank Richter 3 14, Javier Coy-Vergara 15, Giovanna Coceano 16, Edward S Boyden 17, Rory R Duncan 5, Stefan W Hell 6, Marcel A Lauterbach 7, Stephan E Lehnart 8 9, Tobias Moser 10 11, Tiago F Outeiro 12 13, Peter Rehling 14 18, Blanche Schwappach 15, Ilaria Testa 16, Bolek Zapiec 19, Silvio O Rizzoli 20 2
Affiliations
- PMID: 29146773
- PMCID: PMC5753035
- DOI: 10.15252/embj.201695709
Glyoxal as an alternative fixative to formaldehyde in immunostaining and super-resolution microscopy
Katharina N Richter et al. EMBO J. 2018.
Abstract
Paraformaldehyde (PFA) is the most commonly used fixative for immunostaining of cells, but has been associated with various problems, ranging from loss of antigenicity to changes in morphology during fixation. We show here that the small dialdehyde glyoxal can successfully replace PFA Despite being less toxic than PFA, and, as most aldehydes, likely usable as a fixative, glyoxal has not yet been systematically tried in modern fluorescence microscopy. Here, we tested and optimized glyoxal fixation and surprisingly found it to be more efficient than PFA-based protocols. Glyoxal acted faster than PFA, cross-linked proteins more effectively, and improved the preservation of cellular morphology. We validated glyoxal fixation in multiple laboratories against different PFA-based protocols and confirmed that it enabled better immunostainings for a majority of the targets. Our data therefore support that glyoxal can be a valuable alternative to PFA for immunostaining.
Keywords: PFA; fixation; glyoxal; immunocytochemistry; super‐resolution Microscopy.
© 2017 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
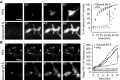
Figure 1. Comparison of cell penetration by PFA and glyoxal
- Speed of propidium iodide (PI) penetration into fibroblasts during 60 min of fixation with either 4% PFA or 3% glyoxal. N = 3 independent experiments. Glyoxal fixation enables PI to penetrate far more rapidly into the cells.
- Speed of FM 1‐43 penetration in similar experiments. The arrowhead points to one example of ongoing endocytosis during PFA fixation. N = 3–4 independent experiments. The general pattern of FM 1‐43 entry was similar to that of propidium iodide. Only the first 10 min are shown, to enable an optimal observation of the kinetics of the first stages of FM 1‐43 entry. The results parallel those obtained with PI: faster penetration during glyoxal fixation.
Data information: Scale bar = 40 μm; **P < 0.01 (two‐sided Student's _t_‐test).
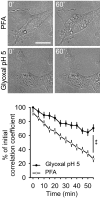
Figure 2. A comparison of morphological changes taking place during fixation with PFA or glyoxal
The changes were visualized by DIC images taken at 5‐min intervals during fixation. The graph shows the correlation of each image to the first frame. N = 50 (PFA) and 54 (glyoxal) cellular regions analyzed, from three independent experiments (mean ± SEM). The higher correlation value indicates that glyoxal preserves the initial cell morphology with higher accuracy than PFA. Scale bar = 20 μm; **P < 0.01 (two‐sided Student's _t_‐test).
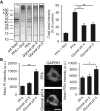
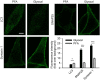
Figure 3. Comparison of protein and RNA fixation by PFA and glyoxal
- SDS–PAGE gel showing rat brain cytoplasm incubated for 60 min with different fixatives. The graph shows the summed intensity of the bands in each lane. Fixed proteins either no longer run into the gel or form only smears. To compare the efficiency of fixation, the bands that survive fixation were summed and were expressed as % of an unfixed control. The intensity of PFA‐fixed samples was significantly higher than that of glyoxal‐fixed samples (N = 5 independent experiments; one‐way ANOVA with post hoc Tukey test). Glut = 0.2% glutaraldehyde.
- Staining of nucleic acids after fixation. The propidium iodide signal in fibroblasts was significantly higher for samples fixed with glyoxal pH 4 (N = 6–8). To test whether the fixed nucleic acids were still available for specific detection, we performed FISH for GAPDH in cultured neurons, using a standard protocol provided by the company Affymetrix. The fluorescence signal of the samples fixed with glyoxal (pH 4) was significantly higher than for PFA‐fixed samples (N = 5–6; two‐sided Student's _t_‐test).
Data information: The graphs show mean values, and error bars represent standard error of the mean. Scale bar = 10 μm; *P < 0.05, **P < 0.01.
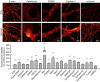
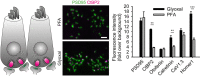
Figure 4. STED imaging of primary hippocampal neurons fixed with either PFA or glyoxal
Strong differences in labeling patterns can be observed. The images are brighter and less “spotty” for the glyoxal‐fixed samples. Structures such as filaments or organelles are more easily detected. Quantification of the fluorescence signal (fold over background) shows that 16 out of 20 stainings are significantly brighter in glyoxal‐fixed samples compared to the PFA‐fixed samples. N = 35–132 objects (mean ± SEM). Scale bar = 6 μm for β‐actin and α‐tubulin and 3 μm for the other proteins. **P < 0.01, ***P < 0.001 (two‐sided Student's _t_‐test for PSD95, Wilcoxon rank‐sum test for all other proteins).
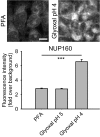
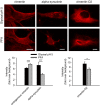
Figure 5. Comparison of immunostaining NUP160 after fixation with either PFA or glyoxal
HeLa cells were stained for the nucleoporin complex protein NUP160 after fixation with either PFA, glyoxal pH 4 or glyoxal pH 5. Fluorescence intensities (fold over background) were compared and are shown in the graph. The quantification of fluorescence signals shows that glyoxal pH 4 fixation allows for significantly brighter stainings. N = 73–156 cells per condition analyzed (mean ± SEM). Scale bar = 10 μm. ***P < 0.001 (Wilcoxon rank‐sum test).
Figure 6. Comparison of immunostained AtT20 cells after fixation with either PFA or glyoxal
AtT20 cells stained for the SNARE proteins syntaxin 1 and SNAP25 and the autophagy marker LC3B were compared with regard to the fluorescence intensity (fold over background) of the stainings. Quantification of the intensity shows that glyoxal fixation allows for significantly brighter stainings of the membrane SNARE proteins. LC3B staining is brighter in PFA‐fixed cells. N = 9–20 cells per condition (mean ± SEM). Scale bar = 5 μm. *P < 0.05, ***P < 0.001 (two‐sided Student's _t_‐test).
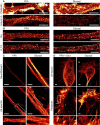
Figure 7. Comparison of immunostained primary hippocampal neurons in STED resolution
- Primary hippocampal neurons were stained for actin and ankyrin G. A comparison between PFA‐ and glyoxal‐fixed samples shows that actin staining with phalloidin works as least as well in both, showing the prominent actin rings. Ankyrin G staining is brighter in PFA‐fixed cells.
- Primary hippocampal neurons were stained for pan‐Nav and Kv7.2. Both stainings seem to work at least as well for glyoxal‐fixed neurons as for PFA‐fixed neurons. Staining of K‐channels shows a slightly more regular pattern in glyoxal‐fixed neurons.
- Primary hippocampal neurons were stained for neurofilament L and beta II spectrin. While the spectrin staining seems to be equally well in both fixation conditions, neurofilament staining is brighter in glyoxal‐fixed cells.
- Growth cones of hippocampal neurons were stained for actin and βIII‐tubulin after either glyoxal or PFA + glutaraldehyde fixation. The latter is a standard fixation used for the co‐labeling of tubulin and actin and is a stronger fixation than normal PFA fixation, which is incompatible with many organelle immunostainings (unlike glyoxal fixation). The filopodia and lamellipodia of the growth cones seem to be well stained for the samples fixed with glyoxal, whereas the samples fixed with PFA and glutaraldehyde seem to have lost some of the finer actin structures. Tubulin seems to be a bit better stained in samples fixed with PFA and glutaraldehyde.
Data information: Scale bars = 1 μm.
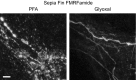
Figure 8. Comparison of immunostained sepia fin after fixation with either PFA or glyoxal
Sepia fin was fixed with the respective fixative and stained for the neuropeptide FMRFamide. A clear change in morphology can be observed between samples fixed with PFA and samples fixed with glyoxal. The former appear broken and swollen, while the glyoxal‐fixed ones appear complete. The effect is presumably due to the different speed of penetration into tissue and/or fixation. Scale bar = 5 μm.
Figure 9. Comparison of immunostained ventricular myocytes fixed 10 min with either PFA or glyoxal
Freshly isolated murine ventricular myocytes were either fixed with 4% PFA or 3% glyoxal and immunostained for caveolin‐3 or ryanodine receptor type 2. Quantification of the fluorescence intensity of the stainings shows significantly brighter stainings for glyoxal‐fixed myocytes. The graph shows mean values, and error bars represent standard deviations. N = 10 (RyR2) and 12 (Cav3) myocytes per condition. Scale bar = 2 μm. ***P < 0.001 (Wilcoxon rank‐sum test).
Figure 10. Comparison of immunostained mouse inner hair cells after fixation with either PFA or glyoxal
Acutely dissected organs of Corti were fixed in the respective fixative and immunostained for inner hair cell proteins and synaptic proteins. The quantification of fluorescence intensity for each staining shows a significant increase in signal‐to‐noise ratio for three target proteins (CtBP2, calretinin, and Homer 1) fixed with glyoxal. None of the stained proteins shows a significant decrease in fluorescence after glyoxal fixation. Representative images show maximum intensity projections from _z_‐stacks of inner hair cell ribbon synapses. N = 5 independent stainings from two animals per condition (PSD95, CtBP2, otoferlin, calretinin) and 10–15 images per condition (CaV1.3 and Homer 1) (mean ± SEM). Scale bar = 2 μm. **P < 0.01, ***P < 0.001 (two‐sided Student's _t_‐test for CtBP2, otoferlin, calretinin and Homer1, Wilcoxon rank‐sum test for PSD95 and CaV1.3).
Figure 11. Comparison of stained vimentin and α‐synuclein in human neuroglioma cells after fixation with either PFA or glyoxal
Cells were fixed with PFA or glyoxal for 10 min and were stained for endogenous vimentin, or for expressed α‐synuclein. A quantification of the staining intensities indicates that glyoxal fixation allows for significantly brighter stainings for alpha‐synuclein, but that PFA was superior for endogenous vimentin (leftmost graph). The fluorescence intensity of vimentin expressed with an mOrange2 tag was also analyzed after fixation with PFA or with glyoxal; the latter allowed more mOrange2 fluorescence to be detected (rightmost graph). N = 29–81 cell regions per condition (mean ± SEM). *P < 0.05, ***P < 0.001 (Wilcoxon rank‐sum test). Scale bar: 10 μm.
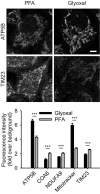
Figure 12. Comparison of immunostainings for mitochondrial proteins after fixation with either PFA or glyoxal
Cells were stained with MitoTracker Orange prior to fixation with the respective fixative and immunostained for the mitochondrial proteins ATP5B, COA6, NDUFA9, and TIM23. Quantification of the staining intensity shows a significant increase of fluorescence (signal over background) for two markers (ATP5B and MitoTracker) after fixation with glyoxal, whereas for the remaining three proteins, immunostainings seem to be more efficient after fixation with PFA, albeit the differences are small. N = 18–128 cells per condition (mean ± SEM). Scale bar = 5 μm. ***P < 0.001 (two‐sided Student's _t_‐test for ATP5B and NDUFA9, Wilcoxon rank‐sum test for all other proteins).
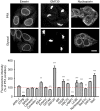
Figure 13. Comparison of immunostained HeLa cells after fixation with either PFA or glyoxal
The fluorescence intensity of a variety of proteins was compared between cells fixed with PFA or glyoxal. Quantification is shown as percentage of signal derived from PFA‐fixed cells. Eight out of the 18 target proteins which were stained show significantly brighter signal when fixed with glyoxal. Only four proteins show significantly reduced staining intensity. For LC3B, the intensity of less than 5 cells was quantified; therefore, single data points were plotted in addition to the bars. N = 3–44 cells per condition (mean ± SEM). Scale bar = 10 μm. **P < 0.01, ***P < 0.001 (Wilcoxon rank‐sum test for APPL1, ATPB, BAG6, caveolin‐1, GATA6 and HSC70, two‐sided Student's _t_‐test for all other proteins).
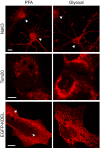
Figure 14. Comparison of immunostained U2OS cells and primary hippocampal neurons after either PFA or glyoxal fixation
Immunostaining of the Na/K ATPase in primary hippocampal neurons shows a different distribution of the protein between PFA‐fixed and glyoxal‐fixed samples. While in PFA‐fixed neurons, the antibody falsely stains the nucleus as well as the cytoplasm (100% of the 82 cells we analyzed), in glyoxal‐fixed neurons the nucleus is devoid of signal, and the membrane appears to be correctly labeled (arrowheads; 100% of the 60 cells we analyzed). U2OS cells were immunostained for mitochondria (Tom20) and ER (EGFP‐KDEL). The fixation and/or staining of mitochondria seems to be comparable in glyoxal and in PFA‐fixed cells. The staining of the ER shows an improved signal‐to‐noise ratio. The signal appears de‐localized from the ER for multiple PFA‐stained cells (25% of 36 analyzed cells, see arrows), while this is rare for the glyoxal‐stained cells (3.4% of 58 analyzed cells). Scale bar = 10 μm.
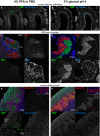
Figure 15. Comparison of mouse tissue staining following either PFA or glyoxal fixation
- A, B
Confocal images showing staining of olfactory marker protein (OMP) and β3‐tubulin along the dorsal aspect of the mouse olfactory epithelium. While sections from both types of fixative show OMP signal in the olfactory sensory neuron somata, their dendrites, and axons, the axon bundles (green arrow) located above the olfactory epithelium exemplify the clear signal‐to‐noise ratio benefits of glyoxal fixation versus that of the PFA‐fixative. Immunostaining with the β3‐tubulin antibody stains the dendrites and axons (blue arrowheads) in both PFA and glyoxal‐fixed tissue, but strong staining of the cilia (blue arrows) can only be observed in the glyoxal‐fixed sections (B). - C, D
Confocal images depicting bundles of axons belonging to olfactory sensory neurons on the path toward the olfactory bulb. Identities of the axons are in part defined by the neuropilin‐1 (Nrp1) and neuropilin‐2 (Nrp2) expression levels, visualized here with antibodies raised against the two proteins. While complementary expression of the two molecules can be seen in the PFA‐fixed sections (C), the glyoxal‐fixed sections (D) exhibit profoundly improved signal‐to‐noise ratios for Nrp‐1 (red arrows), and in the case of Nrp‐2, also the segmentation of the axon bundle into varying levels of Nrp‐2 (green arrows). - E, F
Confocal images of olfactory sensory neuron axons coalescing into glomeruli where they synapse with dendrites of olfactory bulb neurons. The axons of olfactory sensory neurons can be readily visualized with OMP staining (green) in the superficial olfactory nerve layer and terminating in glomeruli located below (green arrows). While sections fixed with either PFA or glyoxal display adequate staining levels, the signal distribution of the PFA‐fixed tissue appears more irregular (E), seemingly lacking the neurofilamentary morphology that appears preserved in the glyoxal‐fixed sections (F). The glomeruli themselves are neuropil structures comprised primarily of olfactory sensory neurons forming synapses with dendrites of mitral/tufted cells as well as dendrites of periglomerular neurons. Immunostaining with vesicular glutamate transporter 2 (VGLUT2) allows visualization of these structures and is easily seen in the glyoxal‐fixed section (F), while it appears the antigen was masked by PFA fixation as no signal above background can be seen in the PFA‐fixed panel (red arrows in E). Note that a different polyclonal antibody for VGLUT2 from the same provider does provide signal with PFA, albeit weaker versus glyoxal (inset). Staining with β3‐tubulin touts the benefits of glyoxal both due to the signal improvements in the case of the mild staining in the axons of the olfactory nerve layer that is only visible in the glyoxal‐fixed tissue, but also in preserving tissue morphology as demonstrated by the dendritic processes inside glomeruli (blue arrows) and in the external plexiform layer located below the glomeruli.
Figure 16. Overview of the results obtained from all immunostainings, in all of the laboratories testing glyoxal
Various cellular targets, ranging from the nucleus to synapses of hippocampal neurons, were tested after fixation with either PFA or glyoxal by us and 11 additional laboratories. Overall, 51 targets were better stained after glyoxal fixation than after PFA fixation, 12 targets were stained worse, and 19 targets were equally well stained.
Similar articles
- Histomorphometric comparison after fixation with formaldehyde or glyoxal.
Wang YN, Lee K, Pai S, Ledoux WR. Wang YN, et al. Biotech Histochem. 2011 Oct;86(5):359-65. doi: 10.3109/10520295.2010.520275. Epub 2010 Sep 21. Biotech Histochem. 2011. PMID: 20854226 Free PMC article. - An optimized fixation method containing glyoxal and paraformaldehyde for imaging nuclear bodies.
Yao RW, Luan PF, Chen LL. Yao RW, et al. RNA. 2021 Jun;27(6):725-733. doi: 10.1261/rna.078671.120. Epub 2021 Apr 12. RNA. 2021. PMID: 33846273 Free PMC article. - PFA is superior to glyoxal in preserving oocyte, embryo, and stem cell proteins evidenced by super-resolution microscopical surveys of epitopes.
Celikkan FT, Mungan C, Sucu M, Uysal F, Kahveci Hayme S, Hayme S, Kuscu N, Ozkavukcu S, Celik-Ozenci C, Can A. Celikkan FT, et al. J Assist Reprod Genet. 2020 Feb;37(2):369-384. doi: 10.1007/s10815-019-01666-9. Epub 2020 Jan 13. J Assist Reprod Genet. 2020. PMID: 31930433 Free PMC article. - Tissue fixation and the effect of molecular fixatives on downstream staining procedures.
Howat WJ, Wilson BA. Howat WJ, et al. Methods. 2014 Nov;70(1):12-9. doi: 10.1016/j.ymeth.2014.01.022. Epub 2014 Feb 21. Methods. 2014. PMID: 24561827 Free PMC article. Review. - Fixation strategies for retinal immunohistochemistry.
Stradleigh TW, Ishida AT. Stradleigh TW, et al. Prog Retin Eye Res. 2015 Sep;48:181-202. doi: 10.1016/j.preteyeres.2015.04.001. Epub 2015 Apr 17. Prog Retin Eye Res. 2015. PMID: 25892361 Free PMC article. Review.
Cited by
- Heterogeneity of late endosome/lysosomes shown by multiplexed DNA-PAINT imaging.
Bond C, Hugelier S, Xing J, Sorokina EM, Lakadamyali M. Bond C, et al. J Cell Biol. 2025 Jan 6;224(1):e202403116. doi: 10.1083/jcb.202403116. Epub 2024 Nov 1. J Cell Biol. 2025. PMID: 39485275 - Reverse engineering of feedforward cortical-Hippocampal microcircuits for modelling neural network function and dysfunction.
Hanssen KS, Winter-Hjelm N, Niethammer SN, Kobro-Flatmoen A, Witter MP, Sandvig A, Sandvig I. Hanssen KS, et al. Sci Rep. 2024 Oct 29;14(1):26021. doi: 10.1038/s41598-024-77157-4. Sci Rep. 2024. PMID: 39472479 Free PMC article. - Strategies for Making High-Performance Artificial Spider Silk Fibers.
Schmuck B, Greco G, Pessatti TB, Sonavane S, Langwallner V, Arndt T, Rising A. Schmuck B, et al. Adv Funct Mater. 2024 Aug 28;34(35):2305040. doi: 10.1002/adfm.202305040. Epub 2023 Oct 10. Adv Funct Mater. 2024. PMID: 39355086 Free PMC article. Review. - In focus in HCB.
Taatjes DJ, Roth J. Taatjes DJ, et al. Histochem Cell Biol. 2024 Oct;162(4):257-258. doi: 10.1007/s00418-024-02319-4. Histochem Cell Biol. 2024. PMID: 39120704 No abstract available. - Spatial Visualization of A-to-I Editing in Cells Using Endonuclease V Immunostaining Assay (EndoVIA).
Quillin AL, Arnould B, Knutson SD, Heemstra JM. Quillin AL, et al. ACS Cent Sci. 2024 Jul 8;10(7):1396-1405. doi: 10.1021/acscentsci.4c00444. eCollection 2024 Jul 24. ACS Cent Sci. 2024. PMID: 39071059 Free PMC article.
References
- Beaudoin GMJ, Lee S‐H, Singh D, Yuan Y, Ng Y‐G, Reichardt LF, Arikkath J (2012) Culturing pyramidal neurons from the early postnatal mouse hippocampus and cortex. Nat Protoc 7: 1741–1754 - PubMed
- Boucher J, Simard É, Froehlich U, D'Orléans‐Juste P, Grandbois M (2015) Using carboxyfluorescein diacetate succinimidyl ester to monitor intracellular protein glycation. Anal Biochem 478: 73–81 - PubMed
- Brunelle JL, Green R (2014) One‐dimensional SDS‐polyacrylamide gel electrophoresis (1D SDS‐PAGE), 1st edn Amsterdam, the Netherlands: Elsevier Inc. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources