Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis - PubMed (original) (raw)
Testosterone therapy in hypogonadal men: a systematic review and network meta-analysis
Jesse Elliott et al. BMJ Open. 2017.
Abstract
Objective: To assess the relative effects of individual testosterone products among hypogonadal men.
Design: Systematic review and network meta-analysis.
Methods: We searched MEDLINE, Embase, Cochrane CENTRAL, and grey literature (25 May 2017) for randomised-controlled trials (RCTs) and non-randomised studies (NRS) that involved hypogonadal men given testosterone replacement therapy (TRT) for ≥3 months. Comparators were placebo, another TRT, or the same product at a different dose. Outcomes were quality of life, depression, libido, erectile function, activities of daily living and testosterone levels, as well as cardiovascular death, myocardial infarction, stroke, prostate cancer, heart disease, diabetes, serious adverse events, withdrawals due to adverse events and erythrocytosis. RCT data were pooled via meta-analysis and network meta-analysis. Risk of bias was assessed using Cochrane's risk of bias tool (RCTs) andScottish Intercollegiate Guidelines Network (SIGN)50 (NRS).
Results: Eighty-seven RCTs and 51 NRS were included. Most were at high or unclear risk of bias, with short treatment duration and follow-up. When compared as a class against placebo, TRT improved quality of life (standardised mean difference (SMD) -0.26, 95% CI -0.41 to -0.11), libido (SMD 0.33, 95% CI 0.16 to 0.50), depression (SMD -0.23, 95% CI -0.44 to -0.01) and erectile function (SMD 0.25, 95% CI 0.10 to 0.41). Most individual TRTs were significantly better than placebo at improving libido (6/10). Only one TRT was better than placebo at improving quality of life, and no individual TRTs improved depression or erectile function. There was no increased risk of adverse events, with the exception of withdrawals due to adverse events with the use of some TRTs.
Conclusion: Despite a class effect of improving quality of life, depression, erectile function and libido, major improvements were not observed with the use of any individual product. We observed no statistically significant increase in the risk of adverse events; however, longer-term high-quality trials are needed to fully assess the risk of harm.
Prospero registration number: CRD42014009963.
Keywords: benefits; cardiovascular-related adverse events; depression; erectile function; harms; libido; network meta-analysis; quality of life; systematic review; testosterone.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: MM has received honoraria for serving on Advisory Boards for Astra Zeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Hoffman La Roche, Novartis, Novo Nordisk and Pfizer, outside the submitted work. BS is a paid information consultant/contractor to the Ottawa Hospital Heart Institute. All other authors have declared no conflicts of interests.
Figures
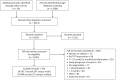
Figure 1
PRISMA flow diagram showing selection of studies. NRS, non-randomised studies; RCT, randomised-controlled trial; TRT, testosterone replacement therapy.
Figure 2
Meta-analysis of the effect of testosterone on quality of life.
Figure 3
Evidence network for quality of life. The size of each circle (node) is proportional to the number of randomly assigned patients and indicates sample size. The number of randomised-controlled trials (RCTs) that contributed to each direct comparison is indicated on the line between nodes. IM, intramuscular injection; TC, testosterone cypionate; TE, testosterone enanthate; TU, testosterone undecanoate.
Figure 4
Odds of cardiovascular death associated with the use of any testosterone product versus placebo.
References
- Summary Safety Review – Testosterone Replacement Products Cardiovascular Risk. 2014. www.hc-sc.gc.ca/dhp-mps/medeff/reviews-examens/testosterone-eng.php (accessed 15 Aug 2016).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources