CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy - PubMed (original) (raw)
Clinical Trial
doi: 10.1038/nm.4441. Epub 2017 Nov 20.
Nirali N Shah 1, Rimas J Orentas 1, Maryalice Stetler-Stevenson 2, Constance M Yuan 2, Sneha Ramakrishna 1, Pamela Wolters 1, Staci Martin 1, Cindy Delbrook 1, Bonnie Yates 1, Haneen Shalabi 1, Thomas J Fountaine 1, Jack F Shern 1, Robbie G Majzner 3, David F Stroncek 4, Marianna Sabatino 4, Yang Feng 5, Dimiter S Dimitrov 5, Ling Zhang 1, Sang Nguyen 1, Haiying Qin 1, Boro Dropulic 6, Daniel W Lee 1, Crystal L Mackall 7
Affiliations
- PMID: 29155426
- PMCID: PMC5774642
- DOI: 10.1038/nm.4441
Clinical Trial
CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy
Terry J Fry et al. Nat Med. 2018 Jan.
Abstract
Chimeric antigen receptor (CAR) T cells targeting CD19 mediate potent effects in relapsed and/or refractory pre-B cell acute lymphoblastic leukemia (B-ALL), but antigen loss is a frequent cause of resistance to CD19-targeted immunotherapy. CD22 is also expressed in most cases of B-ALL and is usually retained following CD19 loss. We report results from a phase 1 trial testing a new CD22-targeted CAR (CD22-CAR) in 21 children and adults, including 17 who were previously treated with CD19-directed immunotherapy. Dose-dependent antileukemic activity was observed, with complete remission obtained in 73% (11/15) of patients receiving ≥1 × 106 CD22-CAR T cells per kg body weight, including 5 of 5 patients with CD19dim or CD19- B-ALL. Median remission duration was 6 months. Relapses were associated with diminished CD22 site density that likely permitted CD22+ cell escape from killing by CD22-CAR T cells. These results are the first to establish the clinical activity of a CD22-CAR in B-ALL, including leukemia resistant to anti-CD19 immunotherapy, demonstrating potency against B-ALL comparable to that of CD19-CAR at biologically active doses. Our results also highlight the critical role played by antigen density in regulating CAR function.
Figures
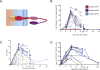
Figure 1. Expansion of CD22 CAR T cells infused following lymphodepleting chemotherapy
A. Percentage of circulating T-cells which express CD22 CAR as measured by flow cytometry. B. Absolute number of circulating CAR T-cells per mcL blood calculated by multiplying the percent CD22 CAR positive by the absolute CD3+ T cells/mcL. C. Copies of integrated CD22-CAR transgene per 100 mcg of DNA obtained from peripheral blood mononuclear cells.
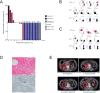
Figure 2. CD22 CAR T cells induce remission in patients with relapsed and refractory pre-B ALL including CD19 CAR-resistant ALL
A. Waterfall plot demonstrating percent change in bone marrow aspirate blast frequency from baseline to day 28 (+/− 4 days) and cytokine release syndrome (CRS) grading in the first 21consecutive patients treated, color coded by dose level. *Asterisks designates a patient with progressive disease (PD) defined as greater than 50% increase in circulating blasts. #Denotes minimal residual disease (MRD) negative complete remission. B. Eradication of CD19dim ALL relapsing after CD19-CAR therapy following infusion of 3×105/kg CD22 CAR T cells in patient #2. Top 3 dot plots show leukemia cell surface antigen expression in bone marrow. Bottom 2 rows of dot plots demonstrate clearance of circulating blasts (top) and CAR expansion (bottom) in blood. C. CAR expansion and clearance of CD19neg CD19-CAR resistant ALL following infusion of 1×106/kg CD22 CAR T cells in patient #15. C. Immunohistochemistry (staining CD79a, a pan-B cell marker) of a bone marrow biopsy of patient #15 demonstrating MRD negative complete remission and B-cell aplasia one month following CD22 CAR T cell infusion. Magnification 200x. D. Serial PET scans showing pre-treatment disease and evolution to full resolution in post-treatment evaluations in patient #13.
Figure 3. Changes in CD22 expression level in a subset of patients following CD22 CAR T cells
A. Change in CD22 expression levels in patients achieving remission following CD22 CAR T cell therapy who subsequently developed relapse. Site density measured by flow cytometry as described in methods.. B. Patient #9 who enrolled with CD19 negative B-ALL, experienced an MRD negative remission following CD22-CAR, with subsequent relapse at 6 months demonstrating emergence of 2 leukemic populations with altered CD22 expression compared to pretreatment. C. Relapse with CD22 negative ALL following MRD negative remission in Patient #15 with pretreatment CD19 negative ALL. Last therapy prior to anti-CD22 CAR was with inotuzumab ozogamicin. At the time of relapse, 23% of T-cells in the marrow and 14.4% of T-cells in the peripheral blood were CD22 CAR positive. D. Patient #11 relapsed 1.5 mos following CD22 CAR infusion with CD22lo B-ALL. Therapy administered immediately prior to anti-CD22 CAR was inotuzumab ozogamicin
Figure 4. CD22 site density limits CD22 CAR functionality
A. Histogram of CD22 expression on CRISPR/Cas9 edited (as described in methods) CD22 negative NALM6 B-ALL lines transduced to express varying levels of CD22. Table shows site density using the same flow cytometry-based assay used to measure CD22 on patient samples and described in supplemental methods. NALM6 refers to the parental cell line. Interferon gamma (B) and IL-2 (C) production by CD22 CAR transduced T cells upon co-culture with NALM6 cell lines expressing varying CD22 site densities. *=p<0.05, ***=p <0.005, ****=p<0.001 by one way analysis of variance (ANOVA). Data shown in B and C is representative of 3 independent experiments. Lines represent means +/− standard error of measurent of triplicate wells. D. Xenograft model (as described in Methods) demonstrating clearance of parental NALM6 by CD22 CAR T cells at a dose of 6×106 per mouse (NOD SCID gamma (NSG)) administered at day 3 following leukemia injection but failure of the same CAR T cells to eradicate NALM6 expressing low CD22 site density despite initial delay in leukemia progression. Representative of 3 independent experiments.
Figure 5. Whole Exome and RNAseq profiling of CD22 in Primary Patient Samples and Patient Derived Xenograft (PDX) recurring in the presence of CD22 CAR Immunotherapeutic Immune pressure
(A) Genome-wide copy number profiling of the primary and relapsed leukemia sample from Patient #5 demonstrate no net ploidy changes across the genome. Interrogation of the CD22 locus demonstrated no exon level alteration in the pre or post treatment sample. (B) Analysis of RNAseq for Patient #5 showed maintenance of the CD22 mRNA. (C) A patient-derived xenograft (PDX) developed from patient #5 was treated in vivo with CD22 CAR which showed tumor response followed by CD22 flow cytometry-negative relapse. (D) Genome-wide copy number and CD22 exon analysis demonstrated genomic stability from the primary tumor with no gains, losses and maintenance of the ploidy status before and after therapy. (E) CD22 transcript levels in the pre and post treatment sample again showed maintenance of consistent mRNA expression.
Figure 6. CD22xCD19 bispecific CAR demonstrates in vitro and in vivo activity against CD19- and CD22- ALL
A. Schematic representation of the CD19xCD22 CAR construct. Interferon gamma production measured by enzyme-linked immun in response to (B) and killing in an in vitro assay measuring loss of GFP-labeled targets of (C) parental NALM6 and CRISPR/Cas9 edited CD19neg and CD22neg (as described in Methods) NALM6 cell lines. In vitro killing of GFP-expressing NALM6 ALL measured by loss of GFP+ cells. Data presented in panels B and C is representative of 2 independent experiments. D. Eradication of NALM6 in NOD SCID gamma (NSG) mice (as described in Methods) by CAR expressing T cells as indicated and is representative of 4 independent experiments.
Similar articles
- Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia.
Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, Han X, Liu Y, Zhang W, Wang C, Zhang Y, Chen M, Yang Q, Wang Y, Han W. Dai H, et al. J Hematol Oncol. 2020 Apr 3;13(1):30. doi: 10.1186/s13045-020-00856-8. J Hematol Oncol. 2020. PMID: 32245502 Free PMC article. Clinical Trial. - CRISPR/Cas9-Engineered Universal CD19/CD22 Dual-Targeted CAR-T Cell Therapy for Relapsed/Refractory B-cell Acute Lymphoblastic Leukemia.
Hu Y, Zhou Y, Zhang M, Ge W, Li Y, Yang L, Wei G, Han L, Wang H, Yu S, Chen Y, Wang Y, He X, Zhang X, Gao M, Yang J, Li X, Ren J, Huang H. Hu Y, et al. Clin Cancer Res. 2021 May 15;27(10):2764-2772. doi: 10.1158/1078-0432.CCR-20-3863. Epub 2021 Feb 24. Clin Cancer Res. 2021. PMID: 33627493 Clinical Trial. - Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation.
Liu S, Deng B, Yin Z, Lin Y, An L, Liu D, Pan J, Yu X, Chen B, Wu T, Chang AH, Tong C. Liu S, et al. Am J Hematol. 2021 Jun 1;96(6):671-679. doi: 10.1002/ajh.26160. Epub 2021 Mar 29. Am J Hematol. 2021. PMID: 33725422 Clinical Trial. - CAR-T Cell Therapy for Acute Lymphoblastic Leukemia: Transforming the Treatment of Relapsed and Refractory Disease.
Pehlivan KC, Duncan BB, Lee DW. Pehlivan KC, et al. Curr Hematol Malig Rep. 2018 Oct;13(5):396-406. doi: 10.1007/s11899-018-0470-x. Curr Hematol Malig Rep. 2018. PMID: 30120708 Review.
Cited by
- Revolutionizing CAR T-Cell Therapies: Innovations in Genetic Engineering and Manufacturing to Enhance Efficacy and Accessibility.
Giorgioni L, Ambrosone A, Cometa MF, Salvati AL, Nisticò R, Magrelli A. Giorgioni L, et al. Int J Mol Sci. 2024 Sep 26;25(19):10365. doi: 10.3390/ijms251910365. Int J Mol Sci. 2024. PMID: 39408696 Free PMC article. Review. - Advances and applications of RNA vaccines in tumor treatment.
Yang R, Cui J. Yang R, et al. Mol Cancer. 2024 Oct 9;23(1):226. doi: 10.1186/s12943-024-02141-5. Mol Cancer. 2024. PMID: 39385255 Free PMC article. Review. - CAR-T cell therapy: Advances in digestive system malignant tumors.
Xu N, Wu Z, Pan J, Xu X, Wei Q. Xu N, et al. Mol Ther Oncol. 2024 Sep 10;32(4):200872. doi: 10.1016/j.omton.2024.200872. eCollection 2024 Dec 19. Mol Ther Oncol. 2024. PMID: 39377038 Free PMC article. Review. - Generating advanced CAR-based therapy for hematological malignancies in clinical practice: targets to cell sources to combinational strategies.
Zhou S, Yang Y, Jing Y, Zhu X. Zhou S, et al. Front Immunol. 2024 Sep 20;15:1435635. doi: 10.3389/fimmu.2024.1435635. eCollection 2024. Front Immunol. 2024. PMID: 39372412 Free PMC article. Review. - DNA vaccines against GPRC5D synergize with PD-1 blockade to treat multiple myeloma.
Neeli P, Maza PAMA, Chai D, Zhao D, Hoi XP, Chan KS, Young KH, Li Y. Neeli P, et al. NPJ Vaccines. 2024 Oct 1;9(1):180. doi: 10.1038/s41541-024-00979-w. NPJ Vaccines. 2024. PMID: 39353958 Free PMC article.
References
- Ram R, et al. Adolescents and young adults with acute lymphoblastic leukemia have a better outcome when treated with pediatric-inspired regimens: systematic review and meta-analysis. Am J Hematol. 2012;87:472–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials