IL-11 is a crucial determinant of cardiovascular fibrosis - PubMed (original) (raw)
. 2017 Dec 7;552(7683):110-115.
doi: 10.1038/nature24676. Epub 2017 Nov 13.
Sivakumar Viswanathan 2, Anissa A Widjaja 2, Wei-Wen Lim 1, Aida Moreno-Moral 2, Daniel M DeLaughter 3, Benjamin Ng 1, Giannino Patone 4, Kingsley Chow 1, Ester Khin 2, Jessie Tan 1, Sonia P Chothani 2, Lei Ye 1, Owen J L Rackham 2, Nicole S J Ko 2, Norliza E Sahib 2, Chee Jian Pua 1, Nicole T G Zhen 1, Chen Xie 1, Mao Wang 2, Henrike Maatz 4, Shiqi Lim 1, Kathrin Saar 4, Susanne Blachut 4, Enrico Petretto 2, Sabine Schmidt 4, Tracy Putoczki 5 6, Nuno Guimarães-Camboa 7, Hiroko Wakimoto 3, Sebastiaan van Heesch 4, Kristmundur Sigmundsson 2, See L Lim 1, Jia L Soon 1 2, Victor T T Chao 1 2, Yeow L Chua 1, Teing E Tan 1, Sylvia M Evans 7 8 9, Yee J Loh 1 10, Muhammad H Jamal 1, Kim K Ong 1 10, Kim C Chua 1, Boon-Hean Ong 1, Mathew J Chakaramakkil 1, Jonathan G Seidman 3, Christine E Seidman 3 11 12, Norbert Hubner 4 13 14 15, Kenny Y K Sin 1 2, Stuart A Cook 1 2 16 17
Affiliations
- PMID: 29160304
- PMCID: PMC5807082
- DOI: 10.1038/nature24676
IL-11 is a crucial determinant of cardiovascular fibrosis
Sebastian Schafer et al. Nature. 2017.
Abstract
Fibrosis is a common pathology in cardiovascular disease. In the heart, fibrosis causes mechanical and electrical dysfunction and in the kidney, it predicts the onset of renal failure. Transforming growth factor β1 (TGFβ1) is the principal pro-fibrotic factor, but its inhibition is associated with side effects due to its pleiotropic roles. We hypothesized that downstream effectors of TGFβ1 in fibroblasts could be attractive therapeutic targets and lack upstream toxicity. Here we show, using integrated imaging-genomics analyses of primary human fibroblasts, that upregulation of interleukin-11 (IL-11) is the dominant transcriptional response to TGFβ1 exposure and required for its pro-fibrotic effect. IL-11 and its receptor (IL11RA) are expressed specifically in fibroblasts, in which they drive non-canonical, ERK-dependent autocrine signalling that is required for fibrogenic protein synthesis. In mice, fibroblast-specific Il11 transgene expression or Il-11 injection causes heart and kidney fibrosis and organ failure, whereas genetic deletion of Il11ra1 protects against disease. Therefore, inhibition of IL-11 prevents fibroblast activation across organs and species in response to a range of important pro-fibrotic stimuli. These results reveal a central role of IL-11 in fibrosis and we propose that inhibition of IL-11 is a potential therapeutic strategy to treat fibrotic diseases.
Conflict of interest statement
The authors declare competing financial interests: details are available in the online version of the paper.
Figures
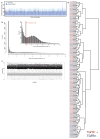
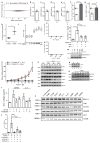
Extended Data Figure 1. RNA-seq of fibrotic cardiac fibroblasts
a, Mapped RNA-seq reads for human fibroblasts (n = 84 biologically independent samples). We aimed to generate at least 60 million reads per sample to assess RNA expression with and without stimulation of primary cardiac fibroblasts with TGFβ1. Fibroblasts were derived from the atria of 84 individuals, resulting in a total of 168 RNA-seq datasets for unstimulated and stimulated cells. After mapping, we used only reads that map to one unique location in the genome to estimate gene expression levels. b, Distribution of mean gene expression across all samples for each gene. We found 12,081 genes to be expressed at log2(TPM + 1) > 2. Genes below this cut-off were not considered in subsequent analyses. c, TPM distribution for each sample after filtering (n = 84). d, Ward clustering of Euclidian distance of RNA-seq samples. Sample gene expression tends to cluster mostly by genotype, indicating a strong genetic effect, beyond the effects of TGFβ1 stimulation.
Extended Data Figure 2. Characterization the TGFβ1-regulated gene, Il-11
a, We measured the amount of activated fibroblasts (ACTA2+ cells) using the Operetta High-content imaging platform and IL-11 transcript levels by RNA-seq (n = 84 biologically independent samples). The increase in IL-11 expression correlated strongly with fibroblast activation in the cohort (ρ = 0.47, 95% confidence interval = 0.28–0.62). ST, stimulated cells (5 ng ml−1, 24 h); BL, baseline cells (unstimulated, 24 h). b, TGFβ1 (5 ng ml−1, 24 h) significantly upregulates IL11 RNA (8.5-fold, P = 6 × 10−218) in human atrial fibroblasts according to RNA-seq analysis (n = 84 biologically independent samples). P values were calculated with DEseq2. c, The change in gene expression was confirmed at the protein level using an ELISA assay to measure IL-11 protein in the supernatant of cardiac fibroblasts (n = 6 biologically independent samples). fc, fold change. Two-tailed Student’s _t_-test. d, RNA-seq-based expression differences in IL11 transcript between unstimulated and TGFβ1-stimulated cardiac fibroblasts were confirmed via RT–qPCR. e, f, The JSD was calculated for all protein-coding genes expressed in activated fibroblasts. Low JSD scores indicate that a gene is highly expressed in stimulated cardiac fibroblasts and lowly expressed in healthy tissues (e; GTEx) or unstimulated, primary cell lines (f; FANTOM). g, h, IL11 RNA expression in TGFβ1-stimulated and unstimulated cardiac fibroblasts (n = 84 biologically independent samples) compared to GTEx (g; tissues from n = 8,723 biologically independent samples) and FANTOM (h; cell types from n = 285 biologically independent samples) databases. g, RNA expression of IL11 in TGFβ1-stimulated cardiac fibroblasts (red) and healthy tissues. h, RNA expression of IL11 in TGFβ1-stimulated cardiac fibroblasts (red) and primary cells. b, c, g, h, Box-and-whisker plots show median (middle line), 25th–75th percentiles (box) and 10th–90th percentiles (whiskers).
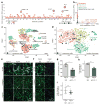
Extended Data Figure 3. Single-cell RNA-seq of fibrotic mouse heart
Single-cell RNA-seq analysis of cells isolated from PlnR9C/+ and wild-type adult left ventricles shown in Fig. 1. a, Cell types were defined according to indicated marker genes. b, Cells were clustered and cell type was determined using the Seurat R package (see Methods). c, Upregulation of Il-11 in the heart in the PlnR9C/+ fibrosis model was confirmed using western blotting. All mice were 18 weeks old and male. d, Subsequently, 1,263 fibroblasts from PlnR9C/+ and wild-type mice were re-clustered using Seurat. WNT signalling, downstream of TGFβ1 in cardiac fibroblast activation, target genes were used in this analysis of the four subsequent clusters of fibroblasts; Il-11+ cells were primarily found in clusters 0 and 1 (16 out of 18 Il-11-expressing cells). Clusters 0 and 1 were also enriched for PlnR9C/+ cells compared to wild-type. Il11ra1 was expressed in all clusters. Cardiac cells were sequenced from n = 1 mouse, experiment was repeated one time with similar results.
Extended Data Figure 4. IL-11 activates fibroblasts and is required for the pro-fibrotic effect of TGFβ1
a, b, High-resolution fluorescence imaging after TGFβ1 or IL-11 treatment (5 ng ml−1, 24 h) of primary cardiac fibroblasts. Immunostaining of nuclei (DAPI, blue), ACTA2 (red) and F-actin (phalloidin, green) indicated that both TGFβ1 and IL-11 activate fibroblast stress fibre formation and increase the number of myofibroblasts in vitro to similar levels. Experiment was repeated four times with similar results. c, Automated quantification of fluorescence (Operetta assay n = 7 measurements per n = 6 independent experiments) of primary atrial fibroblasts reveals significant fibroblast activation and ECM production induced by both TGFβ1 and IL-11 (5 ng ml−1, 24 h). d, In addition, TGFβ1 effects can be reduced with an anti-IL-11 antibody (2 μg ml−1). c, d, Collagen secretion in the supernatant (n = 6 independent experiments) was assessed with Sirius Red. e, Mouse primary fibroblasts were incubated for 24 h with indicated concentrations of recombinant human or mouse IL-11. Fibroblast activation was monitored using the Operetta High-Content Imaging platform and immunostaining for ACTA2. rhIL-11 was found to inefficiently activate mouse fibroblasts (rmIL-11, n = 2, rhIL-11, n = 4 biologically independent samples) compared to rmIL-11; this occurred for rhIL-11 treatment with rhIL-11 from two separate suppliers. f, g, MMP-2 (f) and TIMP-1 (g) concentration in the supernatant (ELISA) of cardiac fibroblasts (n = 4 biologically independent samples) without stimulus (−), with TGFβ1 or IL-11 (5 ng ml−1, 24 h). h, Il-11-neutralizing antibodies (anti-IL-11, 2 μg ml−1) block the increase in MMP-2 and TIMP-1 protein. i, In vitro monolayer scratch wound assay of cardiac fibroblasts. Wound closure was compared between stimulated (TGFβ1 or IL-11; 5 ng ml−1, 24 h) and unstimulated cardiac fibroblasts (n = 5 biologically independent samples) after 24 h. j, Cardiac fibroblasts (n = 3 biologically independent samples) were seeded in collagen gel and the contraction was monitored. The area of contraction is compared between stimulated (TGFβ1 or IL-11; 5 ng ml−1) and unstimulated groups after 72 h. k, Trans-well migration assay. After 24 h of stimulation (TGFβ1 or IL-11; 5 ng ml−1), cardiac fibroblasts (n = 6 biologically independent samples) that crossed the membrane towards either a TGFβ1- or IL-11-containing compartment were colourimetrically quantified and compared to data from unstimulated cells. l–n, Cardiac fibroblasts were incubated with TGFβ1 (5 ng ml−1, 24 h) and indicated amounts of IL11RA:gp130 decoy receptors (l; 33 amino acid (aa) or 50 aa linker peptide), anti-IL11RA antibody (m; 2 μg ml−1) or siRNA pools against IL-11 or IL11RA (n). l–n, Fibroblast activation was monitored via immunostaining for ACTA2 on the Operetta platform. decoy receptors (l): n = 7 measurements per n = 2 independent experiments; anti- IL11RA (m): n = 7 measurements per n = 2 independent experiments; siRNA (n): Operetta assay n = 7 measurements per n = 10 independent experiments. o, Human renal fibroblasts were incubated with TGFβ1 or IL-11 (5 ng ml−1, 24 h) in the presence or absence of anti-IL-11 or an IgG control antibodies (2 μg ml−1 each) for 24 h. ECM was assessed using the Operetta platform by staining for collagen I. Fluorescence was normalized to non-stimulated cells (black). p, These results were confirmed with Sirius red assay of the total collagen in the supernatant. q, rmIl-11 stimulation (5 ng ml−1, 24 h) also activated mouse cardiac and renal fibroblasts. Myofibroblasts and ECM were assessed using the Operetta platform by staining for ACTA2, collagen I or POSTN. Fluorescence was normalized to non-stimulated cells (black). o–q, These experiments were repeated three times with similar results. r, Cardiac fibroblasts analysed on the Operetta high-content imaging platform with immunostaining of ACTA2 after 24 h incubation without stimulus, TGFβ1 (5 ng ml−1, 24h) or TGFβ1 and IL-6-neutralizing antibody (2 μg ml−1, 24h). Automated quantification of fluorescence (Operetta assay n = 7 measurements per n = 6 independent experiments) shows no significant decrease in fibroblast activation using anti-IL-6 antibodies. Data are mean and circles show individual values (e) or mean ± s.d. and circles show individual values (c, d bottom right, f–h, k); box-and-whisker plots (c, d, l–n, r) show median (middle line), 25th–75th percentiles (box) and 10th–90th percentiles (whiskers). Two-tailed Dunnett’s test (c, f, g, i–k), two-tailed Student’s _t_-test (d, h, r) or two-tailed, Sidak-corrected Student’s _t_-test (l–n). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Extended Data Figure 5. IL-11 drives fibrogenic protein expression via non-canonical ERK signalling
a, Genome-wide RNA expression differences of cardiac fibroblasts in response to IL-11 (n = 4 biologically independent samples, 5 ng ml−1, 24 h). Red indicates differentially expressed genes according to DEseq2. Fibrosis gene RNA is not increased by IL-11 treatment. b–e, RT–qPCR experiments for RNA expression of ACTA2 (b), POSTN (c), MMP2 (d) and TIMP1 (e) in response to IL-11 treatment (5 ng ml−1, 24 h) compared to unstimulated cells. IL-11 does not significantly upregulate these genes at the RNA level in cardiac fibroblasts (n = 4 biologically independent samples). f, Sirius red assay reveals significant increase in collagen protein. g, ELISA reveals increase in MMP-2 protein in the supernatant of the samples (shown in Fig. 2b, n = 6 biologically independent samples) that lack a change in RNA transcripts. h, Concentration of IL11RA (ELISA) in the supernatant of cardiac fibroblasts (n = 3 biologically independent samples) after TGFβ1 stimulation (5 ng ml−1, 24 h). i, Cardiac fibroblasts were incubated with increasing concentrations of a fusion protein consisting of IL-11 and IL11RA connected with a linker peptide that recapitulates the features of the IL-11 trans_-signalling complex. Concentrations as low as 200 pg ml−1 significantly activated cardiac fibroblasts as measured using a highcontent imaging platform and staining for ACTA2 expression (Operetta assay n = 7 measurements per n = 4 independent experiments). j, k, rhIL-11 (j) and hyperIL-11 (k) were added at indicated concentrations and subsequently measured using a commercially available IL-11 ELISA (n = 1 independent experiment). The ELISA did not detect rhIL-11 or hyperIL-11. We note that the reactivity to rhIL-11 was variable dependent on batch and provider and rhIL-11 was sometimes detected. However, in all experiments presented in the main figures, we confirmed that the rhIL-11 used was not detectable by the ELISA by additional measurements. This ELISA reliably detected native IL-11 secreted by human fibroblasts. l, Cardiac fibroblasts (n = 3 biologically independent samples) were incubated (8 h) with hyperIL-11 (0.2 ng ml−1) in the presence of absence of the inhibitor of protein translation, cyclohexamide (CHX, 5 μg ml−1), or protein secretion, brefeldin A (BFA, 1 μg ml−1). Both inhibitors block the increase in IL-11 protein in the supernatant in response to hyperIL-11 treatment. m, Western blot and ELISA of IL-11 in cardiac fibroblasts after hyperIL-11, the inhibitor of the Golgi secretory pathway BFA and/or the translation inhibitor CHX treatment shows de novo protein synthesis and canonical secretion of IL-11 after stimulation. n, ELISA (n = 9 biologically independent samples) and RT–qPCR (n = 5 biologically independent samples) assays show an increase in endogenous IL-11 protein but not RNA over time after rhIL-11 (5 ng ml−1) treatment. o, ERK signalling pathway activation by TGFβ1 and IL-11. Western blots show activation of the non-canonical MEK–ERK– RSK cascade in response to IL-11 stimulation of human cardiac fibroblasts. Here the response was greatest at 15 min in the two patients analysed, but more prolonged ERK activation was also seen in additional experiments. p, Downstream substrates of ERK, such as eIF4E, were also phosphorylated by rhIL-11. q, TGFβ1 also activates the ERK pathway. The time course and degree of activation was variable between patients. r, Collagen secretion (Sirius red) from cardiac fibroblasts (control, n = 6; TGFβ1, n = 6; TGFβ1 + U0126, n = 3; TGFβ1 + PD98059, n = 3; IL-11, n = 6; IL-11 + U0126, n = 3; IL-11 + PD98059, n = 3 biologically independent samples) induced by TGFβ1 or IL-11 (5 ng ml−1, 24 h) is reduced by two separate MEK inhibitors (10 μM). s, Western blot of total protein levels of key signalling molecules in fibroblasts after 24 h stimulation with AngII (100 nM), CTGF (50 ng ml−1), EDN1 (250 ng ml−1), bFGF (10 ng ml−1), IL-13 (100 ng ml−1), OSM (100 ng ml−1), PDGF (200 ng ml−1) and TGFβ1 (5 ng ml−1). The corresponding activated protein levels are shown in Fig. 2e. t, siRNA treatment of TGFβ1-stimulated cardiac fibroblasts. RT–qPCR shows SMAD-dependent upregulation of IL11 RNA (control, n = 8; TGFβ1, n = 5; si_TGFB1R, n = 5; si_SMAD2_, n = 4; si_SMAD3_, n = 4 biologically independent samples). Data are mean ± s.d. (b–h, l, n, r, t); box-and-whisker plots (i) show median (middle line), 25th–75th percentiles (box) and 10th–90th percentiles (whiskers). Two-tailed Student’s _t_-test (b–h), two-tailed Dunnett’s test (i, r) or Sidak-corrected, two-tailed Student’s _t_-test (l, t) or one-way ANOVA (n). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Extended Data Figure 6. IL-11 is required for the pro-fibrotic effects of multiple stimuli
a–h, Cardiac fibroblasts were incubated for 24 h with TGFβ1 (a; 5 ng ml−1), CTGF (b; 50 ng ml−1), PDGF (c; 200 ng ml−1), IL-13 (d; 100 ng ml−1), AngII (e; 100 nM), OSM (f; 100 ng ml−1), EDN1 (g; 250 ng ml−1) or bFGF (h; 10 ng ml−1) in the presence or absence of an IL-11-neutralizing antibody (IL-11ab) or IgG control (2 μg ml−1). Cells were stained for ACTA2, collagen I and POSTN to monitor the amount of myofibroblasts and ECM production. High-content imaging and quantification of fluorescence (Operetta assay n = 7 measurements per n = 6 independent experiments for each condition and cellular phenotype) revealed that anti-IL-11 antibodies significantly reduce the pro-fibrotic effect of these stimuli on myofibroblast ratio and ECM production. Two-tailed Dunnett’s test. Box-and-whisker plots show median (middle line), 25th–75th percentiles (box) and 10th–90th percentiles (whiskers).
Extended Data Figure 7. Il-11 acts post-transcriptionally and causes fibrosis in vivo
a, RNA-seq fold change in TGFβ1-regulated genes in Il11ra1+/+ cardiac fibroblasts (n = 3 biologically independent samples) compared to _Il11ra1_−/− cardiac fibroblasts (n = 3 biologically independent samples) after TGFβ1 stimulation (5 ng ml−1, 24 h) are highly correlated. Spearman’s correlation shows that RNA levels of fibrosis genes are upregulated equally in both genotypes. b, Wild-type and knockout fibroblasts (n = 3 biologically independent samples) were incubated with TGFβ1 (5 ng ml−1, 24 h) and RNA-seq was performed to detect differentially expressed genes using DEseq2. All genes regulated by TGFβ1 in wild-type cells are plotted with decreasing − log2(_P_adjusted). The P value of the same genes in stimulated _Il11ra1_−/− cells are plotted to the right. A similar _P_-value distribution suggests that TGFβ1-driven RNA expression changes are still present in the absence of IL-11 signalling showing that loss of Il11ra1 does not influence the TGFβ1-driven transcriptional response. c, d, Primary atrial fibroblasts were prepared from Il11ra1+/+ (c) or _Il11ra1_−/− (d) mice were incubated for 24 h without stimulus or with TGFβ1 (5 ng ml−1), IL-11 (5 ng ml−1) or AngII (100 nM). Cells were stained with antibodies against ACTA2, collagen I or POSTN. Images were taken at low magnification (10×) on the Operetta imaging platform. As shown, fibroblasts from knockout mice do not respond to pro-fibrotic stimuli at the level of pro-fibrotic protein expression. This experiment was repeated four times with similar results. e, Circulating markers of inflammation after rmIl-11 injection (100 μg kg−1 per day, three weeks; n = 14 biologically independent samples). f, Circulating levels of Tgfβ1 (ELISA) after rmIl-11 injection (control, n = 8; Il-11 injection, n = 12 biologically independent samples). g, Collagen content (HPA assay) in atrium (control, n = 7; rmIl-11, n = 10 biologically independent samples) after rmIL-11 treatment. h, The area indicative for collagen deposition was assessed over several fields in n = 4 biologically independent samples and compared between samples from rmIl-11-treated and control mice. i, Representative histological images of the heart and kidney after rmIl-11 injection indicate increased collagen content according to Masson’s trichrome staining. This experiment was repeated three times with similar results. j, RNA expression (RT–qPCR) of fibrosis genes in heart (n = 12 biologically independent samples) and kidney (n = 11 biologically independent samples) after rmIl-11 treatment compared to control. k, RNA expression (RT–qPCR) of fibrosis genes in heart (control, n = 6; Il-11-Tg, n = 3 biologically independent samples) and kidney (control, n = 7; Il-11-Tg, n = 4 biologically independent samples) of tamoxifen-treated Il-11-Tg, Col1a2–CreER and control mice. l, Cardiac fibroblasts were incubated with TGFβ1 or IL-11 (5 ng ml−1) and EdU (10 μM ml−1, 24 h), which was used to detect replicating DNA by fluorescence by automated quantification of images (Operetta assay n = 7 measurements per n = 6 independent experiments). This analysis reveals a significant increase in fibroblast proliferation (EdU+ ells) induced by both TGFβ1 and IL-11. The percentage of EdU+ cells was normalized to the average detected in non-stimulated cells. m, Cells were incubated with TGFβ1 (5 ng ml−1, 24 h) and either an IgG control or anti-IL-11 antibody (2 μg ml−1, 24 h). High-content imaging (Operetta assay n = 7 measurements per n = 6 independent experiments) and quantification of proliferating cells show that anti-IL-11 antibodies significantly reduce the effects of TGFβ1 on fibroblast proliferation. The percentage of EdU+ ells was normalized to the average detected in cells stimulated with TGFβ1 and IgG control. n, Western blots show an increase in Il-11 protein expression in the heart and kidney after tamoxifen treatment in Il-11-Tg, Col1a2–CreER mice. o, Il-11 transgenic mice were crossed with a Col1a2- promoter, tamoxifen-inducible Cre mouse strain (Il-11-Tg). Six-week-old mice were treated with tamoxifen (1 mg per day, 10 consecutive days) to induce Cre-mediated recombination. Likewise, wild-type littermates were injected with tamoxifen for 10 consecutive days as controls. The mice (control creatinine, n = 5; Il-11-Tg creatinine, n = 4; control urea, n = 6; Il-11-Tg urea, n = 4; control Tgfβ1, n = 6; Il-11-Tg Tgfβ1, n = 4 biologically independent samples) were euthanized 14 days after cessation of tamoxifen administration. Serum urea and creatinine increased and indicated renal impairment. We also observed an increase in circulating Tgfβ1 levels. p, Collagen content (HPA assay) in atrium (control, n = 11; Il-11-Tg, n = 4 biologically independent samples) from tamoxifentreated or control Il-11-Tg mice. Data are mean ± s.d. (e, f, h, k, o); box-and-whisker plots (g, j, l, m, p) show median (middle line), 25th–75th percentiles (box) and 10th–90th percentiles (whiskers). Sidak-corrected, two-tailed Student’s _t_-test (e, j, k) or two-tailed Student’s _t_-test (f–h, m, o, p) or Dunnett’s test (l). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
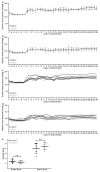
Extended Data Figure 8. Il-11 inhibition does not alter blood pressure after AngII treatment
Il11ra1+/+ wild-type (n = 4 biologically independent samples) and _Il11ra1_−/− knockout (n = 5 biologically independent samples) mice were injected with AngII (2 mg kg−1 per day, 28 days). a, b, Systolic (a) and diastolic (b) blood pressure was measured by in vivo telemetry for one week before and four weeks after the AngII infusion. Systolic (c) and diastolic (d) blood pressure for individual mice. AngII resulted in an increase in blood pressure as expected. The genotype did not have a significant effect on blood pressure. e, Aortic root or arch velocity of the blood. There was no significant difference in the degree of aortic constriction in the TAC model between genotypes (n = 6 biologically independent samples). Two-sided Mann–Whitney _U_-test (e). n.s., not significant. Data are mean ± s.d. (a, b, e).
Extended Data Figure 9. Reduction in collagen deposition in Il11ra1−/− animals is independent of p38 MAPK signalling
Il11ra1+/+ mice (control, n = 13; AngII, n = 10 biologically independent samples) and _Il11ra1_−/− mice (control, n = 5; AngII, n = 7 biologically independent samples) were injected with AngII (100 μg kg−1 per day, three weeks). a, HPA assay of ventricular tissue shows a decrease in collagen deposition in _Il11ra1_−/− mice after AngII infusion. b, Indexed heart weight of wild-type (control, n = 17; AngII, n = 17 biologically independent samples) and _Il11ra1_−/− (control, n = 8; AngII, n = 9 biologically independent samples) mice after AngII injection. c, Indexed heart weight of Il11ra1+/+ (control, n = 4; TAC, n = 6 biologically independent samples) and _Il11ra1_−/− (control, n = 6; TAC, n = 6 biologically independent samples) mice after TAC. d, Kidney weight of Il11ra1+/+ (control, n = 5; folate, n = 8 biologically independent samples) and _Il11ra1_−/− (control, n = 6; folate, n = 5 biologically independent samples) mice three weeks after folate injection (180 mg kg−1). a–d, Sidak-corrected, two-tailed Student’s _t_-test. Data are mean ± s.d. e–g, Western blot of p38 MAPK signalling in tissues of Il11ra1+/+ and _Il11ra1_−/− mice after AngII infusion (e), TAC (f) or folate treatment (g). h, Schematic showing the proposed role of IL-11 fibroblasts. An autocrine loop of IL-11 signalling is required to feed-forward changes in pro-fibrotic mRNA abundances to the protein level by activating translational processes that are ERK-dependent. Blocking this loop limits fibrosis caused by multiple upstream stimuli and fibrosis in preclinical models of heart and kidney disease.
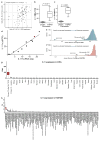
Figure 1. Fibrosis target discovery platform identifies IL-11
a, RNA-seq of primary cardiac fibroblasts (n = 84 biologically independent samples) with or without TGFβ1 treatment (5 ng ml−1, 24 h) and Spearman’s correlation of expression changes with fibroblast activation (Supplementary Table 2). DEseq2 fold change in expression and false-discovery rate (FDR)-adjusted P values are shown. b, RNA expression in transcripts per million (TPM) of IL11RA and IL6R across 512 cell lines from the FANTOM repository. c, Single-cell resolution of cardiac Il11 expression (more than 0 reads per cell). _t_-distributed stochastic neighbour embedding (_t_SNE) analysis clusters cell types of the heart. Il11 expression is highly enriched in fibroblasts. _χ_2 test (P = 5.7 × 10−8). d, _t_SNE analysis of fibroblasts alone shows highest Il-11 expression in ECM-secreting and TGFβ1-activated fibroblasts. _χ_2 test (P = 0.033). c, d, Cardiac cells were sequenced from n = 1 mouse, the experiment was repeated once with similar results. e, f, Representative images (chosen from 42 per condition) of cardiac fibroblasts immunostained for ACTA2, collagen I or periostin (POSTN) after a 24-h incubation without stimulus (control), TGFβ1 or IL-11 (5 ng ml−1) (e) or with TGFβ1 (5 ng ml−1) and an anti-IL-11 neutralizing antibody or an IgG control (2 μg ml−1) (f). g, Cardiac fibroblasts were seeded in collagen gel and the area of contraction determined (n = 3 biologically independent samples) after 72 h. h, Trans-well migration assay (colourimetrically quantified, n = 3 biologically independent samples, 24 h). i, Scratch assay of wound closure in a monolayer of cardiac fibroblasts (n = 5 biologically independent samples) after 24 h. g–i, Two-tailed Student’s _t_-test; data are mean ± s.d.; *P < 0.05; **P < 0.01.
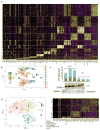
Figure 2. Non-canonical IL-11 signalling drives fibrogenic protein synthesis
a, b, RNA-seq of primary cardiac fibroblasts in response to TGFβ1 (a) or IL-11 (b) (n = 6 biologically independent samples, 5 ng ml−1, 24 h). Red indicates significantly differentially expressed genes (FDR ≤ 0.05, DEseq2). RNA expression of genes associated with fibrosis is not increased by IL-11 treatment. fc, fold change. c, ELISA and quantitative PCR with reverse transcription (RT–qPCR) assays of IL-11 expression (n = 3 biologically independent samples) after hyperIL-11 treatment (0.2 ng ml−1). Benjamini–Hochberg corrected one-way ANOVA; data are mean ± s.d. Inset, western blot of cardiac fibroblast lysates after hyperIL-11 stimulation and brefeldin A (BFA, 1 μg ml−1) treatment indicates canonical secretion of IL-11. d, Cardiac fibroblasts were incubated with TGFβ1, IL-11 (5 ng ml−1) and MEK inhibitors U0126 or PD98059 (10 μM, 24 h). ACTA2+ cells and ECM production was assessed and normalized to non-stimulated cells. e, Western blots of phosphorylated protein (p-) expression of signalling pathways in cardiac fibroblasts in response to various pro-fibrotic stimuli (see also Extended Data Fig. 5). f, ELISA (supernatant, n = 3 biologically independent samples) and RT–qPCR (n = 2 biologically independent samples) of IL-11 expression in cardiac fibroblasts after 24 h stimulation with AngII (100 nM), CTGF (50 ng ml−1), EDN1 (250 ng ml−1), bFGF (10 ng ml−1), IL-13 (100 ng ml−1), OSM (100 ng ml−1), PDGF (200 ng ml−1) and TGFβ1 (5 ng ml−1). Two-tailed Dunnett’s test; Data are mean ± s.d. g, Cardiac fibroblasts were incubated with pro-fibrotic cytokines (24 h) and cardiac fibroblast activation was reduced by anti-IL-11 antibodies (2 μg ml−1; Extended Data Fig. 6). h, Pro-fibrotic proteins (Operetta assay n = 7 measurements per n = 2 independent experiments) are not upregulated in cardiac fibroblasts from _Il11ra1_−/− mice in response to TGFβ1, IL-11 (5 ng ml−1) or AngII (100 nM, 24 h). Experiments were repeated twice with similar results. Two-tailed Dunnett’s test; box-and-whisker plots show median (middle line), 25th–75th percentiles (box) and 10th–90th percentiles (whiskers); **** P < 0.0001; NS, not significant.
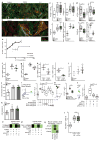
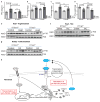
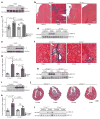
Figure 3. Il-11 causes cardiovascular fibrosis and organ failure
a, Representative histological images of the epicardium with Masson’s trichrome staining and ACTA2 immunostaining in mice with myocardial infarction (MI) treated with rmIL-11 or PBS. b, c, Epicardial thickness of regions distal (b) or proximal (c) to the region of myocardial infarction. a–c, Sham, n = 5; myocardial infarction, n = 6; myocardial infarction + rmIl-11, n = 8 biologically independent samples. d, e, Echocardiography show a decrease in ejection fraction (d) and increase in end-systolic volume (ESV) (e) after myocardial infarction and rmIl-11 treatment (sham, n = 3; myocardial infarction, myocardial infarction + rmIl-11, n = 5 biologically independent samples). b–e, two-tailed, Holm–Sidak-corrected Student’s _t_-test; Data are mean ± s.d. f, Representative histological images (chosen from control, n = 3; rmIl-11 injection, n = 4 biologically independent samples) of tissues from a Col1a1–GFP-reporter mouse after rmIL-11 injection (100 μg kg−1 per day, three weeks). g, Serum urea and creatinine levels after rmIL-11 injection (control urea, n = 8; control creatinine, n = 7; rmIl-11, n = 12 biologically independent samples). h, Reduced ejection fraction (echocardiography) in rmIl-11-treated mice. i, Hydroxyproline assay (HPA) quantifies cardiac and renal collagen content after rmIL-11 treatment. h, i, Control, n = 8; rmIl-11, n = 11 biologically independent samples. j, Representative histological images of Masson’s trichrome staining and ACTA2 immunostaining in the epicardium Il-11-Tg mice. k, HPA indicates cardiac and renal collagen content in Il-11-Tg mice. j, k, Control, n = 12; Il-11-Tg, n = 4 biologically independent samples. i, k, Two-tailed Student’s _t_-test; box-and-whisker plots show median (middle line), 25th–75th percentiles (box) and 10th–90th percentiles (whiskers). l, Reduction in ejection fraction (echocardiography) in Il-11-Tg mice (control, n = 6; Il-11-Tg, n = 4 biologically independent samples). g, h, l, Two-tailed Student’s _t_-test; data are mean ± s.d.; *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Figure 4. Inhibition of Il-11 reduces cardiovascular fibrosis
a, Western blot of cardiac Il-11 after AngIl infusion (2 mg kg−1 per day for 28 days). b, c, Representative histological images (b; Masson’s trichrome staining) and collagen content (c) in the atrium of Il11ra1+/+ (control, n = 12; AngII, n = 9 biologically independent samples) and _Il11ra1_−/− (control, n = 5; AngII, n = 7 biologically independent samples) mice. d, Western blot of cardiac ERK activation after AngII infusion. e, Western blot of cardiac Il-11 after transverse aortic constriction (TAC). f, g, Representative histological images (f; Masson’s trichrome staining) and collagen content. g; HPA of hearts from Il11ra1+/+ (control, n = 4; TAC: n = 6 biologically independent samples) and _Il11ra1_−/− (control, n = 6; TAC, n = 6 biologically independent samples) mice after TAC or sham operations. h, Western blot of cardiac ERK activation after TAC. i, Western blot of renal Il-11 after folate treatment (180 mg kg−1). j, k, Representative histological images (j; Masson’s trichrome staining) and collagen content (k; HPA assay) of kidneys from Il11ra1+/+ (control, n = 5; folate, n = 9 biologically independent samples) and _Il11ra1_−/− (control, n = 6; folate, n = 5 biologically independent samples) mice. c, g, k, Two-tailed, Sidak-corrected Student’s _t_-test; data are mean ± s.d. NS, not significant. l, Western blot of renal ERK activation after folate treatment.
Comment in
- Cardioprotection: IL-11 is a potential therapeutic target in cardiovascular fibrosis.
Fernández-Ruiz I. Fernández-Ruiz I. Nat Rev Cardiol. 2018 Jan;15(1):1. doi: 10.1038/nrcardio.2017.197. Epub 2017 Nov 30. Nat Rev Cardiol. 2018. PMID: 29188810 No abstract available. - Autocrine Activation of Fibroblasts by Induction of IL-11 Expression Is a Common Pathway of Profibrotic Factors.
Mack M. Mack M. Transplantation. 2018 May;102(5):710-711. doi: 10.1097/TP.0000000000002181. Transplantation. 2018. PMID: 29557906 No abstract available.
Similar articles
- Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis.
Widjaja AA, Singh BK, Adami E, Viswanathan S, Dong J, D'Agostino GA, Ng B, Lim WW, Tan J, Paleja BS, Tripathi M, Lim SY, Shekeran SG, Chothani SP, Rabes A, Sombetzki M, Bruinstroop E, Min LP, Sinha RA, Albani S, Yen PM, Schafer S, Cook SA. Widjaja AA, et al. Gastroenterology. 2019 Sep;157(3):777-792.e14. doi: 10.1053/j.gastro.2019.05.002. Epub 2019 May 9. Gastroenterology. 2019. PMID: 31078624 - IL-11 in cardiac and renal fibrosis: Late to the party but a central player.
Corden B, Adami E, Sweeney M, Schafer S, Cook SA. Corden B, et al. Br J Pharmacol. 2020 Apr;177(8):1695-1708. doi: 10.1111/bph.15013. Epub 2020 Feb 22. Br J Pharmacol. 2020. PMID: 32022251 Free PMC article. Review. - Fibroblast-specific IL11 signaling drives chronic inflammation in murine fibrotic lung disease.
Ng B, Dong J, Viswanathan S, Widjaja AA, Paleja BS, Adami E, Ko NSJ, Wang M, Lim S, Tan J, Chothani SP, Albani S, Schafer S, Cook SA. Ng B, et al. FASEB J. 2020 Sep;34(9):11802-11815. doi: 10.1096/fj.202001045RR. Epub 2020 Jul 12. FASEB J. 2020. PMID: 32656894 - Collaborative Regulation of LRG1 by TGF-β1 and PPAR-β/δ Modulates Chronic Pressure Overload-Induced Cardiac Fibrosis.
Liu C, Lim ST, Teo MHY, Tan MSY, Kulkarni MD, Qiu B, Li A, Lal S, Dos Remedios CG, Tan NS, Wahli W, Ferenczi MA, Song W, Hong W, Wang X. Liu C, et al. Circ Heart Fail. 2019 Dec;12(12):e005962. doi: 10.1161/CIRCHEARTFAILURE.119.005962. Epub 2019 Dec 13. Circ Heart Fail. 2019. PMID: 31830829 - Cardiac fibrosis.
Frangogiannis NG. Frangogiannis NG. Cardiovasc Res. 2021 May 25;117(6):1450-1488. doi: 10.1093/cvr/cvaa324. Cardiovasc Res. 2021. PMID: 33135058 Free PMC article. Review.
Cited by
- Harnessing the regenerative potential of interleukin11 to enhance heart repair.
Shin K, Rodriguez-Parks A, Kim C, Silaban IM, Xia Y, Sun J, Dong C, Keles S, Wang J, Cao J, Kang J. Shin K, et al. Nat Commun. 2024 Nov 8;15(1):9666. doi: 10.1038/s41467-024-54060-0. Nat Commun. 2024. PMID: 39516197 Free PMC article. - Molecular Interplay in Cardiac Fibrosis: Exploring the Functions of RUNX2, BMP2, and Notch.
Docshin P, Panshin D, Malashicheva A. Docshin P, et al. Rev Cardiovasc Med. 2024 Oct 16;25(10):368. doi: 10.31083/j.rcm2510368. eCollection 2024 Oct. Rev Cardiovasc Med. 2024. PMID: 39484128 Free PMC article. Review. - Single-Cell RNA Sequencing and Combinatorial Approaches for Understanding Heart Biology and Disease.
Wang L, Jin B. Wang L, et al. Biology (Basel). 2024 Sep 30;13(10):783. doi: 10.3390/biology13100783. Biology (Basel). 2024. PMID: 39452092 Free PMC article. Review. - The role of interleukin-6 classic and trans-signaling and interleukin-11 classic signaling in gastric cancer cells.
Runge J, Garbers C, Lokau J. Runge J, et al. Contemp Oncol (Pozn). 2024;28(2):105-113. doi: 10.5114/wo.2024.142211. Epub 2024 Aug 15. Contemp Oncol (Pozn). 2024. PMID: 39421714 Free PMC article. - Interleukin-11 causes alveolar type 2 cell dysfunction and prevents alveolar regeneration.
Ng B, Huang KY, Pua CJ, Viswanathan S, Lim WW, Kuthubudeen FF, Liu YN, Hii AA, George BL, Widjaja AA, Petretto E, Cook SA. Ng B, et al. Nat Commun. 2024 Oct 2;15(1):8530. doi: 10.1038/s41467-024-52810-8. Nat Commun. 2024. PMID: 39358385 Free PMC article.
References
- Rockey DC, Bell PD, Hill JA. Fibrosis—a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. - PubMed
- Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. - PubMed
- Leaf IA, Duffield JS. What can target kidney fibrosis? Nephrol Dial Transplant. 2017;32(suppl 1):i89–i97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL080494/HL/NHLBI NIH HHS/United States
- DP1 OD006428/OD/NIH HHS/United States
- R01 HL070867/HL/NHLBI NIH HHS/United States
- MC_U120085815/MRC_/Medical Research Council/United Kingdom
- MC_U120097112/MRC_/Medical Research Council/United Kingdom
- DP1 HL117649/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous