Neuroprotective Effect of Ligustilide through Induction of α-Secretase Processing of Both APP and Klotho in a Mouse Model of Alzheimer's Disease - PubMed (original) (raw)
Neuroprotective Effect of Ligustilide through Induction of α-Secretase Processing of Both APP and Klotho in a Mouse Model of Alzheimer's Disease
Xi Kuang et al. Front Aging Neurosci. 2017.
Abstract
Emerging evidence suggests that alpha-processing single transmembrane proteins, amyloid precursor protein (APP) and anti-aging protein Klotho, are likely to be involved in the progression of Alzheimer's disease (AD). The natural phthalide Ligustilide (LIG) has been demonstrated to protect against aging- and amyloid-β (Aβ)-induced brain dysfunction in animal models. The present study is to investigate the effects of LIG on cognitive deficits and metabolism of both APP and Klotho and its underlying mechanism in AD double-transgenic (APP/PS1) mice and cultured human cells. Our results show that treatment with LIG significantly ameliorated memory impairment and Aβ levels and plaques burden. Specifically, LIG might act as a potent enhancer of α-secretase, disintegrin, and metalloprotease 10 (ADAM10), leading to upregulation of alpha-processing of both APP and Klotho and subsequent increases in the levels of both soluble APP fragment (sAPPα) and soluble Klotho (sKL) with inhibition of IGF-1/Akt/mTOR signaling in AD mice and cultured cells. Moreover, the specific ADAM10 inhibitor (G1254023X) effectively reversed LIG-induced alpha-processing of both APP and Klotho in vitro, while Klotho gene knockdown by small interfering RNA significantly blunted LIG-mediated inhibition of IGF-1/Akt/mTOR signaling in vitro. Taken together with the reported neuroprotective effects of both sAPPα and sKL as well as autophagy induction by Akt/mTOR pathway inhibition, our findings suggest that neuroprotection of LIG against AD is associated with induction alpha-processing of APP and Klotho and potential Aβ clearance. Whether LIG might induce Aβ autophagic clearance and the underlying mechanisms need to be further studied.
Keywords: APP; Klotho; Ligustilide (LIG); α-secretase processing; β-amyloid.
Figures
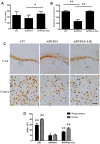
FIGURE 1
Effect of Ligustilide (LIG) on memory impairment and neuronal loss in amyloid precursor protein (APP)/PS1 transgenic mice. C57 mice were treated with vehicle control and APP/PS1 mice treated with either vehicle control or LIG (5 μM) for 14 weeks starting at 8.5 months of age. (A) Effect of LIG on Y-maze test. LIG treatment significantly increased the percentage of alternations used as an index of short-term memory. (B) Effect of LIG on the passive avoidance test. LIG treatment increased the step-down latency in comparison to vehicle-treated APP/PS1 mice. (C) Representative immunostaining of NeuN in the hippocampal CA1 region and cerebral cortex. Scale bar, 25 μm. (D) Quantitative image analysis of NeuN based on the integrated optical density (IOD) of positive immunostaining. The data are expressed as mean ± SEM; n = 12 for behavior tests, n = 4 for immunohistochemistry; ∗p < 0.05, ∗∗p < 0.01.
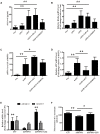
FIGURE 2
Effect of LIG on α-secretase catalytic activity in vitro and in vivo. (A,B) Effect of LIG (1 or 5 μM) on (A) soluble Klotho (sKL) secretion as measured by ELISA and (B) full-length Klotho gene expression as measured by RT-PCR in HEK293T cells. Effect of α-secretase processing was determined by culturing cells in the presence or absence of inhibitors specific to ADAM enzymes (TAPI-O, ADAM17 inhibitor; G1254023X, ADAM10 inhibitor). (C,D) Effect of LIG (1 or 5 μM) on (C) sAPPα secretion as measured by ELISA and (D) full-length APP gene expression as measured by RT-PCR in SH-SY5Ycells expressing hAPP. Effect of α-secretase processing was determined by culturing cells in the presence or absence of ADAM10 inhibitor G1254023X. (E) Effect of LIG on ADAM10 and ADAM17 gene expression in APP/PS1 mice as measured by RT-PCR. (F) Effect of LIG on ADAM enzymatic activity in APP/PS1 mice as measured by the fluorescence of an ADAM peptide-specific fluorescent reporter substrate. The data are expressed as mean ± SEM; n = 4 for RT-PCR, n = 6 for biochemical analysis in mice, and n = 8 for analyses in HEK293T and SH-SY5Y cells; ∗p < 0.05, ∗∗p < 0.01.
FIGURE 3
Effect of LIG on APP processing and amyloid-β generation in APP/PS1 transgenic mice. (A) Representative immunostaining of APP and Aβ42 in the cerebral cortex of mice treated ± LIG or vehicle control. Scale bar, 25 μm. (B) Quantification of immunostaining from (A) based on the IOD of positive immunostaining. (C) Effect of LIG on full-length APP gene expression in APP/PS1 mice as measured by RT-PCR. (D) Effect of LIG on secreted sAPPα in APP/PS1 mice as measured by ELISA. (E,F) Effects of LIG on soluble and insoluble Aβ40 peptides (E) and Aβ42 peptides (F) in brain homogenates of APP/PS1 transgenic mice as measured by ELISA. The data are expressed as mean ± SEM; n = 4 for immunohistochemistry and RT-PCR, n = 6 for biochemical analysis; ∗p < 0.05, ∗∗p < 0.01.
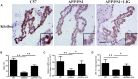
FIGURE 4
Effect of LIG on Klotho expression and processing in APP/PS1 transgenic mice. (A) Representative immunostaining of Klotho in the choroid plexus of APP/PS1 mice in the presence or absence of LIG or vehicle control. Scale bar, 25 μm. (B) Quantification of immunostaining from (A) based on the IOD of positive immunostaining. (C) Effect of LIG on gene expression of Klotho as measured by RT-PCR in APP/PS1 transgenic mice. (D) Effect of LIG on sKL secretion as measured by ELISA in APP/PS1 transgenic mice. The data are expressed as mean ± SEM; n = 4 for immunohistochemistry and RT-PCR, n = 6 for biochemical analysis; ∗p < 0.05, ∗∗p < 0.01.
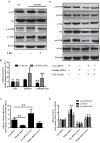
FIGURE 5
Effect of LIG on insulin growth factor 1 (IGF-1)/Akt/m-TOR signaling pathways in vivo and in vitro. (A) Representative western blot analysis of brain homogenates from C57 or APP/PS1 mice treated ± LIG. (B) Quantification of western blot from (A) normalized to control C57 mice. (C) Full-length Klotho gene expression as measured by RT-PCR in HEK293T cells transfected with either control or Klotho-specific siRNA in the presence or absence of LIG. (D) Representative western blot analysis of HEK293T cells from (C) indicating the effect of Klotho inhibition on LIG induced IGF-1/Akt/mTOR signaling. (E) Quantification of western blot from (D) normalized to control C57 mice. The data are expressed as mean ± SEM; n = 4 for western blot and RT-PCR; ∗p < 0.05, ∗∗p < 0.01.
Similar articles
- Ligustilide Ameliorates Memory Deficiency in APP/PS1 Transgenic Mice via Restoring Mitochondrial Dysfunction.
Xu YJ, Mei Y, Qu ZL, Zhang SJ, Zhao W, Fang JS, Wu J, Yang C, Liu SJ, Fang YQ, Wang Q, Zhang YB. Xu YJ, et al. Biomed Res Int. 2018 Jul 10;2018:4606752. doi: 10.1155/2018/4606752. eCollection 2018. Biomed Res Int. 2018. PMID: 30079347 Free PMC article. - Klotho overexpression improves amyloid-β clearance and cognition in the APP/PS1 mouse model of Alzheimer's disease.
Zhao Y, Zeng CY, Li XH, Yang TT, Kuang X, Du JR. Zhao Y, et al. Aging Cell. 2020 Sep 21;19(10):e13239. doi: 10.1111/acel.13239. Online ahead of print. Aging Cell. 2020. PMID: 32964663 Free PMC article. - Ligustilide-loaded liposome ameliorates mitochondrial impairments and improves cognitive function via the PKA/AKAP1 signaling pathway in a mouse model of Alzheimer's disease.
Zhang Q, Zhang X, Yang B, Li Y, Sun XH, Li X, Sui P, Wang YB, Tian SY, Wang CY. Zhang Q, et al. CNS Neurosci Ther. 2024 Mar;30(3):e14460. doi: 10.1111/cns.14460. Epub 2023 Sep 17. CNS Neurosci Ther. 2024. PMID: 37718506 Free PMC article. - Role of the APP non-amyloidogenic signaling pathway and targeting alpha-secretase as an alternative drug target for treatment of Alzheimer's disease.
Bandyopadhyay S, Goldstein LE, Lahiri DK, Rogers JT. Bandyopadhyay S, et al. Curr Med Chem. 2007;14(27):2848-64. doi: 10.2174/092986707782360060. Curr Med Chem. 2007. PMID: 18045131 Review. - Restoring Soluble Amyloid Precursor Protein α Functions as a Potential Treatment for Alzheimer's Disease.
Habib A, Sawmiller D, Tan J. Habib A, et al. J Neurosci Res. 2017 Apr;95(4):973-991. doi: 10.1002/jnr.23823. Epub 2016 Aug 17. J Neurosci Res. 2017. PMID: 27531392 Free PMC article. Review.
Cited by
- AP2S1 regulates APP degradation through late endosome-lysosome fusion in cells and APP/PS1 mice.
Wen QX, Luo B, Xie XY, Zhou GF, Chen J, Song L, Liu Y, Xie SQ, Chen L, Li KY, Xiang XJ, Chen GJ. Wen QX, et al. Traffic. 2023 Jan;24(1):20-33. doi: 10.1111/tra.12874. Epub 2022 Dec 13. Traffic. 2023. PMID: 36412210 Free PMC article. - Reinstating plasticity and memory in a tauopathy mouse model with an acetyltransferase activator.
Chatterjee S, Cassel R, Schneider-Anthony A, Merienne K, Cosquer B, Tzeplaeff L, Halder Sinha S, Kumar M, Chaturbedy P, Eswaramoorthy M, Le Gras S, Keime C, Bousiges O, Dutar P, Petsophonsakul P, Rampon C, Cassel JC, Buée L, Blum D, Kundu TK, Boutillier AL. Chatterjee S, et al. EMBO Mol Med. 2018 Nov;10(11):e8587. doi: 10.15252/emmm.201708587. EMBO Mol Med. 2018. PMID: 30275019 Free PMC article. - The fibroblast growth factor-Klotho axis at molecular level.
Sun F, Liang P, Wang B, Liu W. Sun F, et al. Open Life Sci. 2023 Oct 27;18(1):20220655. doi: 10.1515/biol-2022-0655. eCollection 2023. Open Life Sci. 2023. PMID: 37941788 Free PMC article. Review. - Klotho as Potential Autophagy Regulator and Therapeutic Target.
Zhou H, Pu S, Zhou H, Guo Y. Zhou H, et al. Front Pharmacol. 2021 Oct 19;12:755366. doi: 10.3389/fphar.2021.755366. eCollection 2021. Front Pharmacol. 2021. PMID: 34737707 Free PMC article. Review. - The Chinese herbal formula Fuzheng Quxie Decoction attenuates cognitive impairment and protects cerebrovascular function in SAMP8 mice.
Wang F, Feng J, Yang Y, Liu J, Liu M, Wang Z, Pei H, Wei Y, Li H. Wang F, et al. Neuropsychiatr Dis Treat. 2018 Nov 9;14:3037-3051. doi: 10.2147/NDT.S175484. eCollection 2018. Neuropsychiatr Dis Treat. 2018. PMID: 30519025 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous