Radical SAM Enzymes in the Biosynthesis of Ribosomally Synthesized and Post-translationally Modified Peptides (RiPPs) - PubMed (original) (raw)
Review
Radical SAM Enzymes in the Biosynthesis of Ribosomally Synthesized and Post-translationally Modified Peptides (RiPPs)
Alhosna Benjdia et al. Front Chem. 2017.
Abstract
Ribosomally-synthesized and post-translationally modified peptides (RiPPs) are a large and diverse family of natural products. They possess interesting biological properties such as antibiotic or anticancer activities, making them attractive for therapeutic applications. In contrast to polyketides and non-ribosomal peptides, RiPPs derive from ribosomal peptides and are post-translationally modified by diverse enzyme families. Among them, the emerging superfamily of radical SAM enzymes has been shown to play a major role. These enzymes catalyze the formation of a wide range of post-translational modifications some of them having no counterparts in living systems or synthetic chemistry. The investigation of radical SAM enzymes has not only illuminated unprecedented strategies used by living systems to tailor peptides into complex natural products but has also allowed to uncover novel RiPP families. In this review, we summarize the current knowledge on radical SAM enzymes catalyzing RiPP post-translational modifications and discuss their mechanisms and growing importance notably in the context of the human microbiota.
Keywords: RiPPs; enzyme mechanism; iron sulfur; iron-sulfur proteins; natural products; radical AdoMet; radical SAM; ribosomally synthesized and post-translationally modified peptides.
Figures
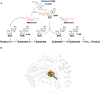
Figure 1
(A) General mechanism of radical SAM enzymes. The radical SAM [4Fe-4S] clusters interacts with _S_-adenosyl-L-methionine (SAM). After the reductive SAM cleavage, the 5′-dA radical species is formed and generally abstracts a substrate H-atom. A radical substrate intermediate is formed which, after subsequent rearrangements, lead to the product. In most of the cases, SAM is used as a co-substrate (right pathway). However, several enzymes recycle SAM during catalysis (left pathway). (B) Structure of a radical SAM enzyme (Spore photoproduct lyase, PDB:4FHD, Benjdia et al., 2012) showing the radical SAM [4Fe-4S] center (in orange and yellow spheres) in interaction with the SAM cofactor.
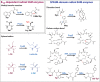
Figure 2
Structures of RiPPs post-translationally modified by radical SAM enzymes. In red are highlighted the modifications catalyzed by radical SAM enzymes. Subtilosin A (thioether bonds), bottromycin A (methylations), thiostrepton A (methylation) (the quinaldic acid moiety is indicated by red dashed lines), epipeptide (epimerization), and polytheonamide A (epimerization and methylations).
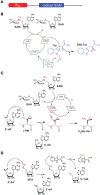
Figure 3
Proposed mechanisms for class B (B12-dependent) and class C radical SAM enzymes. (A) General architecture of B12-dependent radical SAM enzymes. (B) Proposed mechanism for TsrM, an _sp_2-hybridized carbon methyltransferase. (C) Proposed mechanism for PoyC, an _sp_3-hybridized carbon methyltransferase. In contrast to TsrM, PoyC catalyzes SAM homolytic cleavage likely to abstract a substrate Cβ H-atom. This intermediate is likely to react with a methyl radical species leading to the formation of Cβ methyl-valine. In both mechanisms, Cob(II)alamin is likely formed. After further reduction, Cob(I)alamin can react with SAM to regenerate methyl-cobalamin for the next catalytic cycle. (D) Proposed mechanism for NosN, a class C methyltransferase. NosN uses two molecules of SAM to generate 5′-dA radical and 5′-methylthioadenosine (MTA). The 5′-dA radical abstracts an H-atom from the methyl group of MTA leading to the addition of the radical intermediate to the C-4 of the indolyl substrate. After C-S bond cleavage, 5′-thioadenosine (5′-TdA) and the methylated indole product are released.
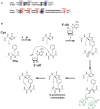
Figure 4
Thioether bond formation by radical SAM enzymes. (A) Sequence alignment of AlbA and anSME showing the cysteine residues involved in the coordination of the [4Fe-4S] clusters present in the SPASM-domain. Cysteine residues involved in the auxiliary cluster I (Aux I) and auxiliary cluster II (Aux II) are indicated in blue and red, respectively. Numbers indicate the respective positions of the cysteines in the protein sequences of AlbA and anSME. (B) Proposed mechanism for AlbA. AlbA catalyzes H-atom abstraction on Cα-atom. The carbon-centered radical rearranges leading to the formation of an _N_-acyliminium intermediate. This intermediate is quenched by the thiolate group of a cysteine residue resulting in the formation of a Cα-thioether bond. The auxiliary clusters I and II are proposed to serve as an electron conduit.
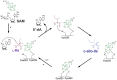
Figure 5
Proposed mechanism for YydG, a radical SAM epimerase. Similarly to AlbA (Figure 4B), YydG catalyzes Cα H-atom abstraction leading to the loss of the amino acid stereochemistry. An enzyme cysteine residue (Cys223) provides an H-atom to the carbon-centered radical intermediate, resulting in peptide epimerization. The additional [4Fe-4S] cluster located in the _C_-terminus end of the protein, likely assists the quenching of the thiyl radical formed during the reaction and regenerates the cysteine H-atom donor, for the next catalytic cycle.
Figure 6
Post-translational modifications catalyzed by Radical SAM enzymes on RiPPs.
Similar articles
- Structural and mechanistic basis for RiPP epimerization by a radical SAM enzyme.
Kubiak X, Polsinelli I, Chavas LMG, Fyfe CD, Guillot A, Fradale L, Brewee C, Grimaldi S, Gerbaud G, Thureau A, Legrand P, Berteau O, Benjdia A. Kubiak X, et al. Nat Chem Biol. 2024 Mar;20(3):382-391. doi: 10.1038/s41589-023-01493-1. Epub 2023 Dec 29. Nat Chem Biol. 2024. PMID: 38158457 - Radical SAM Enzymes and Ribosomally-Synthesized and Post-translationally Modified Peptides: A Growing Importance in the Microbiomes.
Benjdia A, Berteau O. Benjdia A, et al. Front Chem. 2021 Jul 19;9:678068. doi: 10.3389/fchem.2021.678068. eCollection 2021. Front Chem. 2021. PMID: 34350157 Free PMC article. Review. - Bioinformatic Atlas of Radical SAM Enzyme-Modified RiPP Natural Products Reveals an Isoleucine-Tryptophan Crosslink.
Clark KA, Seyedsayamdost MR. Clark KA, et al. J Am Chem Soc. 2022 Oct 5;144(39):17876-17888. doi: 10.1021/jacs.2c06497. Epub 2022 Sep 21. J Am Chem Soc. 2022. PMID: 36128669 - Insights into the catalysis of a lysine-tryptophan bond in bacterial peptides by a SPASM domain radical _S_-adenosylmethionine (SAM) peptide cyclase.
Benjdia A, Decamps L, Guillot A, Kubiak X, Ruffié P, Sandström C, Berteau O. Benjdia A, et al. J Biol Chem. 2017 Jun 30;292(26):10835-10844. doi: 10.1074/jbc.M117.783464. Epub 2017 May 5. J Biol Chem. 2017. PMID: 28476884 Free PMC article. - Accessing and exploring the unusual chemistry by radical SAM-RiPP enzymes.
Guo Q, Morinaka BI. Guo Q, et al. Curr Opin Chem Biol. 2024 Aug;81:102483. doi: 10.1016/j.cbpa.2024.102483. Epub 2024 Jun 24. Curr Opin Chem Biol. 2024. PMID: 38917731 Review.
Cited by
- Darobactin Substrate Engineering and Computation Show Radical Stability Governs Ether versus C-C Bond Formation.
Woodard AM, Peccati F, Navo CD, Jiménez-Osés G, Mitchell DA. Woodard AM, et al. J Am Chem Soc. 2024 May 22;146(20):14328-14340. doi: 10.1021/jacs.4c03994. Epub 2024 May 10. J Am Chem Soc. 2024. PMID: 38728535 Free PMC article. - Genome Mining for New Enzyme Chemistry.
Nguyen DT, Mitchell DA, van der Donk WA. Nguyen DT, et al. ACS Catal. 2024 Mar 12;14(7):4536-4553. doi: 10.1021/acscatal.3c06322. eCollection 2024 Apr 5. ACS Catal. 2024. PMID: 38601780 Free PMC article. Review. - Hydrogen-Deuterium Exchange Mass Spectrometry Identifies Local and Long-Distance Interactions within the Multicomponent Radical SAM Enzyme, PqqE.
Zhu W, Iavarone AT, Klinman JP. Zhu W, et al. ACS Cent Sci. 2024 Jan 17;10(2):251-263. doi: 10.1021/acscentsci.3c01023. eCollection 2024 Feb 28. ACS Cent Sci. 2024. PMID: 38435514 Free PMC article. - Discovery and engineering of ribosomally synthesized and post-translationally modified peptide (RiPP) natural products.
Li H, Ding W, Zhang Q. Li H, et al. RSC Chem Biol. 2023 Nov 21;5(2):90-108. doi: 10.1039/d3cb00172e. eCollection 2024 Feb 7. RSC Chem Biol. 2023. PMID: 38333193 Free PMC article. Review. - Structural and mechanistic basis for RiPP epimerization by a radical SAM enzyme.
Kubiak X, Polsinelli I, Chavas LMG, Fyfe CD, Guillot A, Fradale L, Brewee C, Grimaldi S, Gerbaud G, Thureau A, Legrand P, Berteau O, Benjdia A. Kubiak X, et al. Nat Chem Biol. 2024 Mar;20(3):382-391. doi: 10.1038/s41589-023-01493-1. Epub 2023 Dec 29. Nat Chem Biol. 2024. PMID: 38158457
References
- Arnison P. G., Bibb M. J., Bierbaum G., Bowers A. A., Bugni T. S., Bulaj G., et al. . (2013). Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 30, 108–160. 10.1039/C2NP20085F - DOI - PMC - PubMed
- Banerjee R. V., Matthews R. G. (1990). Cobalamin-dependent methionine synthase. FASEB J. 4, 1450–1459. - PubMed
- Barr I., Latham J. A., Iavarone A. T., Chantarojsiri T., Hwang J. D., Klinman J. P. (2016). Demonstration that the radical S-adenosylmethionine (SAM) enzyme PqqE catalyzes de novo carbon-carbon cross-linking within a peptide substrate PqqA in the presence of the peptide chaperone PqqD. J. Biol. Chem. 291, 8877–8884. 10.1074/jbc.C115.699918 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources