Gut reactions: How the blood-brain barrier connects the microbiome and the brain - PubMed (original) (raw)
Review
Gut reactions: How the blood-brain barrier connects the microbiome and the brain
Aric F Logsdon et al. Exp Biol Med (Maywood). 2018 Jan.
Abstract
A growing body of evidence indicates that the microbiome interacts with the central nervous system (CNS) and can regulate many of its functions. One mechanism for this interaction is at the level of the blood-brain barriers (BBBs). In this minireview, we examine the several ways the microbiome is known to interact with the CNS barriers. Bacteria can directly release factors into the systemic circulation or can translocate into blood. Once in the blood, the microbiome and its factors can alter peripheral immune cells to promote interactions with the BBB and ultimately with other elements of the neurovascular unit. Bacteria and their factors or cytokines and other immune-active substances released from peripheral sites under the influence of the microbiome can cross the BBB, alter BBB integrity, change BBB transport rates, or induce release of neuroimmune substances from the barrier cells. Metabolic products produced by the microbiome, such as short-chain fatty acids, can cross the BBB to affect brain function. Through these and other mechanisms, microbiome-BBB interactions can influence the course of diseases as illustrated by multiple sclerosis. Impact statement The connection between the gut microbiome and central nervous system (CNS) disease is not fully understood. Host immune systems are influenced by changes to the microbiota and offers new treatment strategies for CNS disease. Preclinical studies provide evidence of changes to the blood-brain barrier when animals are subject to experimental gut infection or when the animals lack a normal gut microbiome. The intestine also contains a barrier, and bacterial factors can translocate to the blood and interact with host immune cells. These metastatic bacterial factors can signal T-cells to become more CNS penetrant, thus providing a novel intervention for treating CNS disease. Studies in humans show the therapeutic effects of T-cell engineering for the treatment of leukemia, so perhaps a similar approach for CNS disease could prove effective. Future research should begin to define the bacterial species that can cause immune cells to differentiate and how these interactions vary amongst CNS disease models.
Keywords: Microbiome; T-cell; blood–brain barrier; immune system; multiple sclerosis.
Figures
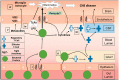
Figure 1.
The microbiome communicates with immune cells throughout the body and can affect the blood–brain barrier (BBB) and CNS function. The gut lumen is constantly exposed to bacterial factors from the outside environment. Disruption of the gut epithelial barrier may permit the unregulated translocation of gut microbes into the lamina propria (i). Bacterial factors can infiltrate the GALT, and the blood lumen, where they interact with various immune cells, including T-cells (ii). Certain bacterial factors can stimulate effector-type T-cell differentiation. Regulatory T-cells survey the GALT, blood, and CSF and changes to the local microbiome can promote T-cell brain infiltration (iii). Circulating bacterial factors can upregulate inflammatory cytokine levels, affect BBB integrity and promote neuroinflammation. LPSs are produced by bacterial factors and can act on endothelial TLRs to promote neuroinflammation and CNS disease (iv). Bacterial metabolites can upregulate tight junction proteins and improve BBB integrity (v). Metabolites can also cross the BBB to impact glial cells and neuroinflammation. The role of the microbiome on pericytes remains unclear (vi). CSF: cerebral spinal fluid; CNS: central nervous system; GALT: gut-associated lymphoid tissues; LPS: lipopolysaccharide; TLRs: toll-like receptors.
Similar articles
- The gut microbiome and microbial translocation in multiple sclerosis.
Mirza A, Mao-Draayer Y. Mirza A, et al. Clin Immunol. 2017 Oct;183:213-224. doi: 10.1016/j.clim.2017.03.001. Epub 2017 Mar 9. Clin Immunol. 2017. PMID: 28286112 Review. - Focus on the gut-brain axis: Multiple sclerosis, the intestinal barrier and the microbiome.
Camara-Lemarroy CR, Metz LM, Yong VW. Camara-Lemarroy CR, et al. World J Gastroenterol. 2018 Oct 7;24(37):4217-4223. doi: 10.3748/wjg.v24.i37.4217. World J Gastroenterol. 2018. PMID: 30310254 Free PMC article. - Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases.
Ghaisas S, Maher J, Kanthasamy A. Ghaisas S, et al. Pharmacol Ther. 2016 Feb;158:52-62. doi: 10.1016/j.pharmthera.2015.11.012. Epub 2015 Nov 26. Pharmacol Ther. 2016. PMID: 26627987 Free PMC article. Review. - Microbiota-gut-brain axis: Interaction of gut microbes and their metabolites with host epithelial barriers.
Bhattarai Y. Bhattarai Y. Neurogastroenterol Motil. 2018 Jun;30(6):e13366. doi: 10.1111/nmo.13366. Neurogastroenterol Motil. 2018. PMID: 29878576 Review. - Unhealthy gut, unhealthy brain: The role of the intestinal microbiota in neurodegenerative diseases.
Spielman LJ, Gibson DL, Klegeris A. Spielman LJ, et al. Neurochem Int. 2018 Nov;120:149-163. doi: 10.1016/j.neuint.2018.08.005. Epub 2018 Aug 14. Neurochem Int. 2018. PMID: 30114473 Review.
Cited by
- Protection of Fecal Microbiota Transplantation in a Mouse Model of Multiple Sclerosis.
Li K, Wei S, Hu L, Yin X, Mai Y, Jiang C, Peng X, Cao X, Huang Z, Zhou H, Ma G, Liu Z, Li H, Zhao B. Li K, et al. Mediators Inflamm. 2020 Aug 5;2020:2058272. doi: 10.1155/2020/2058272. eCollection 2020. Mediators Inflamm. 2020. PMID: 32831634 Free PMC article. - Gut microbes in central nervous system development and related disorders.
Gan Y, Chen Y, Zhong H, Liu Z, Geng J, Wang H, Wang W. Gan Y, et al. Front Immunol. 2024 Jan 26;14:1288256. doi: 10.3389/fimmu.2023.1288256. eCollection 2023. Front Immunol. 2024. PMID: 38343438 Free PMC article. Review. - Intestinal Surgery Contributes to Acute Cerebellar Ataxia Through Gut Brain Axis.
Yu J, Fan Y, Wang L, Huang Y, Xia J, Ding L, Wu CF, Lu X, Ma G, Kim S, Zheng G, Guo H, Zhang G. Yu J, et al. Front Neurol. 2019 Sep 20;10:995. doi: 10.3389/fneur.2019.00995. eCollection 2019. Front Neurol. 2019. PMID: 31616359 Free PMC article. - Towards Improvements for Penetrating the Blood-Brain Barrier-Recent Progress from a Material and Pharmaceutical Perspective.
He Q, Liu J, Liang J, Liu X, Li W, Liu Z, Ding Z, Tuo D. He Q, et al. Cells. 2018 Mar 23;7(4):24. doi: 10.3390/cells7040024. Cells. 2018. PMID: 29570659 Free PMC article. Review. - Unveiling the interplay between NSAID-induced dysbiosis and autoimmune liver disease in children: insights into the hidden gateway to autism spectrum disorders. Evidence from ex vivo, in vivo, and clinical studies.
Mohamed DI, Abo Nahas HH, Elshaer AM, El-Waseef DAEA, El-Kharashi OA, Mohamed SMY, Sabry YG, Almaimani RA, Almasmoum HA, Altamimi AS, Ibrahim IAA, Alshawwa SZ, Jaremko M, Emwas AH, Saied EM. Mohamed DI, et al. Front Cell Neurosci. 2023 Oct 31;17:1268126. doi: 10.3389/fncel.2023.1268126. eCollection 2023. Front Cell Neurosci. 2023. PMID: 38026692 Free PMC article.
References
- Banks WA. The blood-brain barrier as a regulatory interface in the gut-brain axes. Physiol Behav 2006; 89:472–6 - PubMed
- Grossman MI. Neural and hormonal regulation of gastrointestinal function: an overview. Annu Rev Physiol 1979; 41:27–33 - PubMed
- Pearse AG. The diffuse neuroendocrine system and the apud concept: related “endocrine” peptides in brain, intestine, pituitary, placenta, and anuran cutaneous glands. Med Biol 1977; 55:115–25 - PubMed
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog Neurobiol 1998; 54:1–18 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources