Endothelial cell-surface tissue transglutaminase inhibits neutrophil adhesion by binding and releasing nitric oxide - PubMed (original) (raw)
Endothelial cell-surface tissue transglutaminase inhibits neutrophil adhesion by binding and releasing nitric oxide
Thung-S Lai et al. Sci Rep. 2017.
Abstract
Nitric oxide (NO) produced by endothelial cells in response to cytokines displays anti-inflammatory activity by preventing the adherence, migration and activation of neutrophils. The molecular mechanism by which NO operates at the blood-endothelium interface to exert anti-inflammatory properties is largely unknown. Here we show that on endothelial surfaces, NO is associated with the sulfhydryl-rich protein tissue transglutaminase (TG2), thereby endowing the membrane surfaces with anti-inflammatory properties. We find that tumor necrosis factor-α-stimulated neutrophil adherence is opposed by TG2 molecules that are bound to the endothelial surface. Alkylation of cysteine residues in TG2 or inhibition of endothelial NO synthesis renders the surface-bound TG2 inactive, whereas specific, high affinity binding of S-nitrosylated TG2 (SNO-TG2) to endothelial surfaces restores the anti-inflammatory properties of the endothelium, and reconstitutes the activity of endothelial-derived NO. We also show that SNO-TG2 is present in healthy tissues and that it forms on the membranes of shear-activated endothelial cells. Thus, the anti-inflammatory mechanism that prevents neutrophils from adhering to endothelial cells is identified with TG2 S-nitrosylation at the endothelial cell-blood interface.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
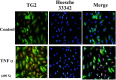
Figure 1
Immunofluorescent staining of TG2 in TNFα-treated HUVEC cells. HUVEC cells were grown and treated with or without TNFα (10 ng/ml) for 5 hours. The cells were non-permeabilized, fixed with 4% paraformaldehyde and stained with mouse monoclonal anti-TG2 (TG100) followed by donkey anti-mouse IgG (Fitc conjugated, shown in Green). The fluorescent signal was visualized using fluorescent microscopy. The nuclei were stained with Hoesche 33342 (shown in blue).
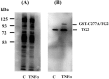
Figure 2
Cell surface biotinylation of cell surface proteins identifies endogenous and exogenous TG2. HUVEC confluent monolayers were incubated with GST-TG2/C277A (50 nM) during the last hour of a 5 hr TNFα (10 ng/ml) treatment. Total cell lysates including membrane fractions were isolated after cell surface proteins biotinylation as described under Materials and Methods. (A) Total cell lysates (15 μg) were separated by SDS-PAGE and transferred to PVDF membrane. Blots were developed using Streptavidin-HRP and bands were visualized using ECL chemiluminescence as described under Materials and Methods. (B) Streptavidin bead pull-down of biotinylated proteins from total cell lysates derived from (A) were performed as described under Materials and Methods. The bound biotinylated proteins were eluted from streptavidin-beads by incubating with SDS-PAGE loading buffer with heating at 95 °C for 4-min. Half of the eluted samples were loaded on a SDS-PAGE and transferred to PVDF membrane. Blots were developed using TG2 mouse monoclonal antibody (cub7402) and goat vs mouse IgG conjugated with HRP and bands were visualized using ECL chemiluminescence.
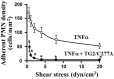
Figure 3
TG2/C277A inhibits neutrophil adhesion to TNFα-activated endothelium. HUVEC monolayers were treated with TG2/C277A (10 nM) during the last hour of a 5 hr TNFα (10 ng/ml) treatment. After infusion of neutrophils (PMN), cultures were exposed to shear stress at the values indicated, and adherent PMNs were then counted. Data are presented as means ± SEM. TNFα, n = 5; TNFα/TG2/C277A, n = 4. p < 0.05, TNFα vs. TNFα/TG2/C277A at all shear stress values. The statistical significance at each shear stress was analyzed using student t-tests. Asterisks denote significant differences. The p-values at each shear stress are as followings: 0.5 dyn/cm2, p = 0.023902; 1 dyn/cm2, p = 0.010764; 2.0 dyn/cm2, p = 0.007297; 4.0 dyn/cm2, p = 0.008728; 8.0 dyn/cm2, p = 0.015503; 20 dyn/cm2, p = 0.010764.
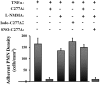
Figure 4
Inhibition of PMN adhesion to endothelial cells is NO dependent. Inhibition of NO synthase (NOS) with L-NMMA prevents inhibition by TG2/C277A of PMN adhesion to endothelial cells pretreated with TNFα, and exogenously S-nitrosylated TG2/C277A inhibits PMN adhesion to TNFα-activated PMNs in the presence of L-NMMA (150 μM). Alkylated TG2/C277A (iodo-TG2/C277A) also prevents inhibition of PMN adhesion. TNF-activated endothelial cells were treated with TG2/C277A, iodo-TG2/C277A or S-nitrosylated TG2/C277A (50 nM, 1 hr) and exposed to flow for 20 min prior to infusion of PMNs. Wall shear stress (τω) = 1 dyn/cm2. Data are presented as means ± SEM, n ≥ 3. *p < 0.05 vs. TNFα. Statistical analysis was performed with a single-factor ANOVA using shear stress = 1.0 dyn/cm2 in all groups: TNFα vs. TNFα/C277A/TG2 (p = 0.002); TNFα/TG277A vs. TNFα/L-NMMA/C277A/TG2 (not nitrosylated) (p = 0.003); TNFα/L-NMMA/SNO-C277A/TG2 vs. TNFα/L-NMMA/C277A/TG2 (not nitrosylated) (p = 0.00044).
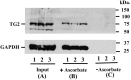
Figure 5
Endogenous TG2 is robustly S-nitrosylated in Mouse Tissues. SNO-TG2 isolated from three separate mouse kidneys (designated as 1, 2 and 3). 10 μg samples were loaded on to each lane for immunoblot analysis. (A) 0.5% of total input (B). Proteins eluted from ascorbate treated samples (+ascorbate) = SNO-proteins. (C). Proteins eluted from untreated samples (−ascorbate) = controls. SNO-modified TG2 was eluted from thiopropyl-Sepharose beads and analyzed by immunoblots using mouse monoclonal antibody against TG2 (cub7402, ThermoFisher Scientific) as described under Materials and Methods. GAPDH, a prototypic SNO-modified protein (detected using rabbit monoclonal antibody Abcam, Ab181602) is included as a control.
Similar articles
- Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase.
Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Lai TS, et al. Biochemistry. 2001 Apr 24;40(16):4904-10. doi: 10.1021/bi002321t. Biochemistry. 2001. PMID: 11305905 - Monocyte-derived tissue transglutaminase in multiple sclerosis patients: reflecting an anti-inflammatory status and function of the cells?
Sestito C, Brevé JJP, van Eggermond MCJA, Killestein J, Teunissen CE, van Rossum J, Wilhelmus MMM, Drukarch B, van den Elsen PJ, van Dam AM. Sestito C, et al. J Neuroinflammation. 2017 Dec 21;14(1):257. doi: 10.1186/s12974-017-1035-y. J Neuroinflammation. 2017. PMID: 29268771 Free PMC article. - Nitric oxide regulates tissue transglutaminase localization and function in the vasculature.
Jandu SK, Webb AK, Pak A, Sevinc B, Nyhan D, Belkin AM, Flavahan NA, Berkowitz DE, Santhanam L. Jandu SK, et al. Amino Acids. 2013 Jan;44(1):261-9. doi: 10.1007/s00726-011-1090-0. Epub 2011 Oct 8. Amino Acids. 2013. PMID: 21984378 Free PMC article. - Cellular functions of tissue transglutaminase.
Nurminskaya MV, Belkin AM. Nurminskaya MV, et al. Int Rev Cell Mol Biol. 2012;294:1-97. doi: 10.1016/B978-0-12-394305-7.00001-X. Int Rev Cell Mol Biol. 2012. PMID: 22364871 Free PMC article. Review. - Transglutaminase 2 in the balance of cell death and survival.
Fésüs L, Szondy Z. Fésüs L, et al. FEBS Lett. 2005 Jun 13;579(15):3297-302. doi: 10.1016/j.febslet.2005.03.063. Epub 2005 Apr 7. FEBS Lett. 2005. PMID: 15943974 Review.
Cited by
- Unraveling the Immunopathological Landscape of Celiac Disease: A Comprehensive Review.
Patt YS, Lahat A, David P, Patt C, Eyade R, Sharif K. Patt YS, et al. Int J Mol Sci. 2023 Oct 23;24(20):15482. doi: 10.3390/ijms242015482. Int J Mol Sci. 2023. PMID: 37895160 Free PMC article. Review. - NETosis Drives Blood Pressure Elevation and Vascular Dysfunction in Hypertension.
Krishnan J, Hennen EM, Ao M, Kirabo A, Ahmad T, de la Visitación N, Patrick DM. Krishnan J, et al. Circ Res. 2024 May 24;134(11):1483-1494. doi: 10.1161/CIRCRESAHA.123.323897. Epub 2024 Apr 26. Circ Res. 2024. PMID: 38666386 Free PMC article. - S-Nitrosylation of Tissue Transglutaminase in Modulating Glycolysis, Oxidative Stress, and Inflammatory Responses in Normal and Indoxyl-Sulfate-Induced Endothelial Cells.
Lin CJ, Chiu CY, Liao EC, Wu CJ, Chung CH, Greenberg CS, Lai TS. Lin CJ, et al. Int J Mol Sci. 2023 Jun 30;24(13):10935. doi: 10.3390/ijms241310935. Int J Mol Sci. 2023. PMID: 37446114 Free PMC article. - How Long Are Reperfusion Therapies Beneficial for Patients after Stroke Onset? Lessons from Lethal Ischemia Following Early Reperfusion in a Mouse Model of Stroke.
Nakagomi T, Tanaka Y, Nakagomi N, Matsuyama T, Yoshimura S. Nakagomi T, et al. Int J Mol Sci. 2020 Sep 2;21(17):6360. doi: 10.3390/ijms21176360. Int J Mol Sci. 2020. PMID: 32887241 Free PMC article. Review. - 3β-Hydroxy-5β-hydroxy-B-norcholestane-6β-carboxaldehyde (SEC-B) Induces Proinflammatory Activation of Human Endothelial Cells Associated with Nitric Oxide Production and Endothelial Nitric Oxide Synthase/Caveolin-1 Dysregulation.
Nasoni MG, Benedetti S, Crinelli R, Palma F, Canonico B, Monittola F, Zerbinati C, Iuliano L, Luchetti F. Nasoni MG, et al. Antioxidants (Basel). 2022 Jun 10;11(6):1148. doi: 10.3390/antiox11061148. Antioxidants (Basel). 2022. PMID: 35740044 Free PMC article.
References
- Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J. 1995;9:866–873. - PubMed
- Smith CW, Marlin SD, Rothlein R, Toman C, Anderson DC. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989;83:2008–2017. doi: 10.1172/JCI114111. - DOI - PMC - PubMed
- Arfors KE, et al. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987;69:338–340. - PubMed
- Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL091876/HL/NHLBI NIH HHS/United States
- R01 HL126900/HL/NHLBI NIH HHS/United States
- R01 HL095463/HL/NHLBI NIH HHS/United States
- R01 HL059130/HL/NHLBI NIH HHS/United States
- P01 HL075443/HL/NHLBI NIH HHS/United States
- F32 CA074520/CA/NCI NIH HHS/United States
- R01 CA040355/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources