Interferon-Stimulated Genes as Enhancers of Antiviral Innate Immune Signaling - PubMed (original) (raw)
Review
Interferon-Stimulated Genes as Enhancers of Antiviral Innate Immune Signaling
Keaton M Crosse et al. J Innate Immun. 2018.
Abstract
The ability of a host to curb a viral infection is heavily reliant on the effectiveness of an initial antiviral innate immune response, resulting in the upregulation of interferon (IFN) and, subsequently, IFN-stimulated genes (ISGs). ISGs serve to mount an antiviral state within a host cell, and although the specific antiviral function of a number of ISGs has been characterized, the function of many of these ISGs remains to be determined. Recent research has uncovered a novel role for a handful of ISGs, some of them directly induced by IFN regulatory factor 3 in the absence of IFN itself. These ISGs, most with potent antiviral activity, are also able to augment varying arms of the innate immune response to viral infection, thereby strengthening this response. This new understanding of the role of ISGs may, in turn, help the recent advancement of novel therapeutics aiming to augment innate signaling pathways in an attempt to control viral infection and pathogenesis.
Keywords: Innate immunity; Interferon; Interferon-stimulated genes; Virus.
© 2017 The Author(s) Published by S. Karger AG, Basel.
Figures
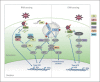
Fig. 1
When a virus enters a cell, viral PAMPS are recognized by pattern recognition receptors, and activate a number of signaling cascades that result in the production of type I and III interferon (IFN) and inflammatory cytokines to induce an antiviral state. These pathways are augmented and enhanced by a number of IFN-stimulated genes (ISGs). Protein kinase R (PKR) is a double-stranded RNA receptor pivotal in the activation of MAVS (both following MDA5 activation and independently of MDA5 and RIG-I activation). Zinc-finger antiviral protein S (ZAP-S) enhances RIG-I ATPase activity. Tripartite motif 21 (TRIM21) enhances innate immune signaling in 2 ways: (1) it detects the Fc portion of the antibody bound to nonenveloped viruses entering the cytosol and catalyzes the synthesis of K63-linked polyubiquinated (K63 Ub) chains to activate IRFs and NF-κB, and induce a type I IFN response independently of RIG-I and cGAS; (2) it recruits the proteasome instigating premature virion uncoating, exposing PAMPS to RIG-I and cGAS. TRIM56 acts as a scaffold protein promoting IRF3 activation by enhancing the efficient interaction of STING and TBK1 and via an interaction with TRIF. Viperin enhances the K63-linked ubiquitination of IRAK1 by promoting an interaction between IRAK1 and TRAF6. DDX60 can bind dsRNA, and it associates with both RIG-I and MDA5 to enhance their activation.
Fig. 2
Protein domains for interferon (IFN)-stimulated genes (ISGs) able to positively augment the type I IFN response. PKR, protein kinase R; ZAP, zinc-finger antiviral protein; ZAP-L, long ZAP isoform; ZAP-S, short ZAP isoform; TRIM, tripartite motif; DDX60, DExD/H box helicase 60; PARP, poly(ADP-ribose) polymerase; RING, really interesting new gene; SAM, S-adenosyl methionine; WWE, W and E residues; aa, amino acids.
Similar articles
- LYAR Suppresses Beta Interferon Induction by Targeting Phosphorylated Interferon Regulatory Factor 3.
Yang C, Liu X, Cheng T, Xiao R, Gao Q, Ming F, Jin M, Chen H, Zhou H. Yang C, et al. J Virol. 2019 Oct 15;93(21):e00769-19. doi: 10.1128/JVI.00769-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31413131 Free PMC article. - Emerging roles of interferon-stimulated genes in the innate immune response to hepatitis C virus infection.
Wong MT, Chen SS. Wong MT, et al. Cell Mol Immunol. 2016 Jan;13(1):11-35. doi: 10.1038/cmi.2014.127. Epub 2014 Dec 29. Cell Mol Immunol. 2016. PMID: 25544499 Free PMC article. Review. - Fish interferon-stimulated genes: The antiviral effectors.
Poynter SJ, DeWitte-Orr SJ. Poynter SJ, et al. Dev Comp Immunol. 2016 Dec;65:218-225. doi: 10.1016/j.dci.2016.07.011. Epub 2016 Jul 19. Dev Comp Immunol. 2016. PMID: 27451256 Review. - Emerging Role of Interferon-Induced Noncoding RNA in Innate Antiviral Immunity.
Min J, Liu W, Li J. Min J, et al. Viruses. 2022 Nov 23;14(12):2607. doi: 10.3390/v14122607. Viruses. 2022. PMID: 36560611 Free PMC article. Review. - Broadly Antiviral Activities of TAP1 through Activating the TBK1-IRF3-Mediated Type I Interferon Production.
Zhao J, Li R, Li Y, Chen J, Feng F, Sun C. Zhao J, et al. Int J Mol Sci. 2021 Apr 28;22(9):4668. doi: 10.3390/ijms22094668. Int J Mol Sci. 2021. PMID: 33925089 Free PMC article.
Cited by
- Endogenous ZAP is associated with altered Zika virus infection phenotype.
Le NPK, Singh PP, Sabir AJ, Trus I, Karniychuk U. Le NPK, et al. Virol J. 2024 Nov 9;21(1):285. doi: 10.1186/s12985-024-02557-x. Virol J. 2024. PMID: 39522048 Free PMC article. - Genetic variants regulating the immune response improve the prediction of COVID-19 severity provided by clinical variables.
Delgado-Wicke P, Fernández de Córdoba-Oñate S, Roy-Vallejo E, Alegría-Carrasco E, Rodríguez-Serrano DA, Lamana A, Montes N, Nicolao-Gómez A, Carracedo-Rodríguez R, Marcos-Jiménez A, Díaz-Fernández P, Galván-Román JM, Rabes-Rodríguez L, Sanz-Alba M, Álvarez-Rodríguez J, Villa-Martí A, Rodríguez-Franco C, Villapalos-García G, Zubiaur P, Abad-Santos F, de Los Santos I, Gomariz RP, García-Vicuña R, Muñoz-Calleja C, González-Álvaro I, Fernández-Ruiz E; PREDINMUN-COVID Group. Delgado-Wicke P, et al. Sci Rep. 2024 Sep 5;14(1):20728. doi: 10.1038/s41598-024-71476-2. Sci Rep. 2024. PMID: 39237611 Free PMC article. - Application of Proteomics Technology Based on LC-MS Combined with Western Blotting and Co-IP in Antiviral Innate Immunity.
Liang W, Zhu Z, Zheng C. Liang W, et al. Methods Mol Biol. 2025;2854:93-106. doi: 10.1007/978-1-0716-4108-8_11. Methods Mol Biol. 2025. PMID: 39192122 - Differential immunometabolic responses to Delta and Omicron SARS-CoV-2 variants in golden syrian hamsters.
Rajaiah R, Pandey K, Acharya A, Ambikan A, Kumar N, Guda R, Avedissian SN, Montaner LJ, Cohen SM, Neogi U, Byrareddy SN. Rajaiah R, et al. iScience. 2024 Jul 14;27(8):110501. doi: 10.1016/j.isci.2024.110501. eCollection 2024 Aug 16. iScience. 2024. PMID: 39171289 Free PMC article. - Influenza, SARS-CoV-2, and Their Impact on Chronic Lung Diseases and Fibrosis: Exploring Therapeutic Options.
Soni S, Antonescu L, Ro K, Horowitz JC, Mebratu YA, Nho RS. Soni S, et al. Am J Pathol. 2024 Oct;194(10):1807-1822. doi: 10.1016/j.ajpath.2024.06.004. Epub 2024 Jul 18. Am J Pathol. 2024. PMID: 39032604 Review.
References
- Helbig KJ, Beard MR. The role of viperin in the innate antiviral response. J Mol Biol. 2014;426:1210–1219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical