Prophylactic Active Tau Immunization Leads to Sustained Reduction in Both Tau and Amyloid-β Pathologies in 3xTg Mice - PubMed (original) (raw)
Prophylactic Active Tau Immunization Leads to Sustained Reduction in Both Tau and Amyloid-β Pathologies in 3xTg Mice
Hameetha Rajamohamedsait et al. Sci Rep. 2017.
Abstract
Amyloid-β (Aβ) and tau pathologies are intertwined in Alzheimer's disease, and various immunotherapies targeting these hallmarks are in clinical trials. To determine if tau pathology influences Aβ burden and to assess prophylactic benefits, 3xTg and wild-type mice received tau immunization from 2-6 months of age. The mice developed a high IgG titer that was maintained at 22 months of age. Pronounced tau and Aβ pathologies were primarily detected in the subiculum/CA1 region, which was therefore the focus of analysis. The therapy reduced histopathological tau aggregates by 70-74% overall (68% in males and 78-86% in females), compared to 3xTg controls. Likewise, western blot analysis revealed a 41% clearance of soluble tau (38-76% in males and 48% in females) and 42-47% clearance of insoluble tau (47-58% in males and 49% in females) in the immunized mice. Furthermore, Aβ burden was reduced by 84% overall (61% in males and 97% in females). These benefits were associated with reductions in microgliosis and microhemorrhages. In summary, prophylactic tau immunization not only prevents tau pathology but also Aβ deposition and related pathologies in a sustained manner, indicating that tau pathology can promote Aβ deposition, and that a short immunization regimen can have a long-lasting beneficial effect.
Conflict of interest statement
E.M.S. is an inventor on patents on tau immunotherapy and related diagnostics that are assigned to New York University. This technology is licensed to and is being co-developed with H. Lundbeck A/S.
Figures
Figure 1
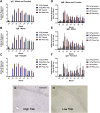
The tau immunogen Tau379–408[P-Ser396, 404] elicits a robust and sustained antibody response. (A–C) IgG response towards the immunogen was strong and long-lasting, and comparable in males vs. females. It was strong at T1 (1 week after the 3rd immunization), peaked at T2 (2 months after the 4th and last immunization) and remained strong thereafter (T3-Tf: 5, 8, 11, and 16 months after the 4th immunization). Higher IgG levels were detected in wt mice compared to 3xTg mice. (D–F) IgM response was not as strong as the IgG response but showed a similar pattern as the IgG response except that the IgM response was comparable in the wt vs. the 3xTg mice. (G,H) Plasma (1:100) obtained from a high titer mouse at the end of the study (Tf) stained tau aggregates in a control 3xTg mouse, whereas plasma from a low titer control mouse did not. Scale bar: 125 μm.
Figure 2
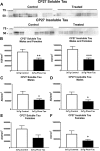
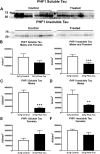
Tau immunization prevents phospho-tau histopathology. (A–D) PHF1 immunostaining revealed extensive tau pathology in the hippocampus (A: 5X objective, B: 20X objective) of 3xTg mice, that was greatly reduced in immunized mice (C,D). (E–H) Only background staining was seen in the wt mice. (I–K) Quantitative analysis of the PHF1 staining revealed significant reduction in tau aggregates in the combined group (I: 74%, p = 0.0008) as well as in males (J: 68%, p = 0.0437) and females (K: 78%, p = 0.0120) analyzed separately. Scale bar: 250 μm (A), 125 µm (B). *p < 0.05; ***p < 0.001 compared to 3xTg control. See text for exact p-values.
Figure 3
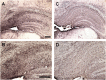
Tau immunization prevents conformational tau histopathology. (A–D) MC1 immunostaining revealed extensive tau pathology in the hippocampus (A: 5X objective, B: 20X objective) of 3xTg mice, that was greatly decreased in immunized 3xTg mice (C-D). (E–H) Only background staining was seen in the wt mice. (I–K) Quantitative analysis of the MC1 staining revealed significant reduction in tau aggregates in the combined group (I: 70%, p = 0.0057) as well as in females (K: 86%, p = 0.0070). Scale bar: 250 μm (A), 125 µm (B). **p < 0.01 compared to 3xTg control. See text for exact p-values.
Figure 4
Tau immunization decreases soluble and insoluble human tau protein. (A,B) Representative blots are shown in A. Note that lanes that appear empty are from mice that did not express human tau and were, therefore, omitted from all analysis. Western blot analysis revealed that soluble and insoluble CP27-reactive human tau were reduced in the immunized 3xTg mice (soluble tau: 41%, p = 0.0103; insoluble tau: 47%, p = 0.0008). (C–F) Comparable tau reductions were observed in males (soluble tau: 38%, p = 0.0322; insoluble tau: 47%, p = 0.0165) vs. females (soluble tau: 48%, p = 0.0616; insoluble tau: 49%, p = 0.0477). *p < 0.05; **p < 0.01; ***p < 0.001 compared to 3xTg control. See text for exact p-values.
Figure 5
Tau immunization reduces soluble and insoluble phospho-tau protein. (A,B) Representative blots are shown in A. Western blot analysis revealed that insoluble PHF1-reactive human tau was decreased in the immunized 3xTg mice (42%, p = 0.0003). (C,D) Both soluble (76%, p = 0.0001) and insoluble tau (58%, p = 0.0018) were decreased in the males but not in the females (E,F). **p < 0.01; ***p < 0.001 compared to 3xTg control. See text for exact p-values.
Figure 6
Tau immunization diminishes Aβ burden. (A–D) Aβ immunostaining revealed extensive Aβ plaque burden in subiculum region of the hippocampus (A: 5X objective, B: 20X objective) of 3xTg mice, that was greatly reduced in immunized mice (C,D). (E–H) Only background staining was seen in the wt mice. (I–K) Quantitative analysis of the Aβ staining revealed significant reduction in Aβ plaque burden in the combined group (I: 84%, p < 0.0001) as well as in males (J: 61%, p = 0.0033) and females (K: 97%, p = 0.0001) analyzed separately. Scale bar: 250 μm (A), 125 µm (B). **p < 0.01; ****p < 0.0001 compared to 3xTg control. See text for exact p-values.
Figure 7
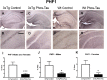
3xTg mice have more astrogliosis than wt mice. (A–D) GFAP immunostaining revealed extensive astrogliosis in subiculum region of the hippocampus (A: 5X objective, B: 10X objective) of 3xTg mice, that was much less in WT mice (C,D). Scale bar: 250 μm.
Figure 8
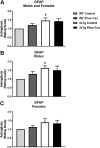
3xTg mice have more astrogliosis than wt mice. (A–C) Semi-quantitative analysis of GFAP immunoreactivity in the subiculum revealed that 3xTg mice had more astrogliosis than wt mice (p = 0.0139) that was also significantly increased in the males (p = 0.0286). However, astrogliosis was not significantly affected by the tau immunotherapy in the combined group (A) or in the males (B) and females (C) analyzed separately. #p < 0.05 compared to wt control. See text for exact p-values.
Figure 9
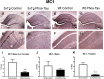
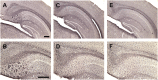
Tau immunization reduces microgliosis in 3xTg mice to wt levels. (A–F) Iba1 immunostaining revealed extensive microgliosis in the subiculum region of the hippocampus in 3xTg mice (A: 5X objective; B: 10X objective) that was greatly reduced in tau immunized mice (C,D) rendering these animals indistinguishable from wt mice (E,F). Scale bar: 250 μm.
Figure 10
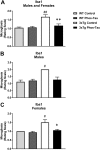
Tau immunization reduces microgliosis in 3xTg mice to wt levels. (A–C) Semi-quantitative analysis of Iba1 immunoreactivity in the subiculum revealed that 3xTg mice had more microgliosis than wt mice (males and females: p = 0.0013; males: p = 0.0286; females: p = 0.0310), and the tau immunotherapy reduced microgliosis in 3xTg mice to wt levels (males and females: p = 0.0056; males: p = 0.0699; females: p = 0.0294). #p < 0.05; ##p < 0.01 compared to wt control. *p < 0.05; **p < 0.01 compared to 3xTg control. See text for exact p-values.
Figure 11
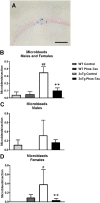
Tau immunization diminishes microbleeds in 3xTg mice to wt levels. (A–D) A few microbleeds (blue) were primarily detected in the hippocampus (A: 20X objective) of the 3xTg control mice (males and females: p = 0.0088 compared to wt control; females: p = 0.0165), and their numbers were significantly reduced in the tau immunized 3xTg mice (males and females: p = 0.0095; females: p = 0.0078) to wt levels. Scale bar: 125 μm. #p < 0.05; ##p < 0.01 compared to wt control. **p < 0.01 compared to 3xTg control. See text for exact p-values.
Similar articles
- Tau passive immunization inhibits not only tau but also Aβ pathology.
Dai CL, Tung YC, Liu F, Gong CX, Iqbal K. Dai CL, et al. Alzheimers Res Ther. 2017 Jan 10;9(1):1. doi: 10.1186/s13195-016-0227-5. Alzheimers Res Ther. 2017. PMID: 28073379 Free PMC article. - Immunotherapy to improve cognition and reduce pathological species in an Alzheimer's disease mouse model.
Herline K, Prelli F, Mehta P, MacMurray C, Goñi F, Wisniewski T. Herline K, et al. Alzheimers Res Ther. 2018 Jun 18;10(1):54. doi: 10.1186/s13195-018-0384-9. Alzheimers Res Ther. 2018. PMID: 29914551 Free PMC article. - Fyn knock-down increases Aβ, decreases phospho-tau, and worsens spatial learning in 3×Tg-AD mice.
Minami SS, Clifford TG, Hoe HS, Matsuoka Y, Rebeck GW. Minami SS, et al. Neurobiol Aging. 2012 Apr;33(4):825.e15-24. doi: 10.1016/j.neurobiolaging.2011.05.014. Epub 2011 Jul 7. Neurobiol Aging. 2012. PMID: 21741124 Free PMC article. - Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?
Merino JJ, Muñetón-Gómez V, Alvárez MI, Toledano-Díaz A. Merino JJ, et al. Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review. - Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease?
Dunys J, Valverde A, Checler F. Dunys J, et al. J Biol Chem. 2018 Oct 5;293(40):15419-15428. doi: 10.1074/jbc.R118.003999. Epub 2018 Aug 24. J Biol Chem. 2018. PMID: 30143530 Free PMC article. Review.
Cited by
- Multipronged diagnostic and therapeutic strategies for Alzheimer's disease.
Ramesh M, Govindaraju T. Ramesh M, et al. Chem Sci. 2022 Oct 14;13(46):13657-13689. doi: 10.1039/d2sc03932j. eCollection 2022 Nov 30. Chem Sci. 2022. PMID: 36544728 Free PMC article. Review. - Structural characterization of monoclonal antibodies targeting C-terminal Ser404 region of phosphorylated tau protein.
Chukwu JE, Congdon EE, Sigurdsson EM, Kong XP. Chukwu JE, et al. MAbs. 2019 Apr;11(3):477-488. doi: 10.1080/19420862.2019.1574530. Epub 2019 Feb 26. MAbs. 2019. PMID: 30794086 Free PMC article. - Immunotherapy targeting plasma ASM is protective in a mouse model of Alzheimer's disease.
Choi BJ, Park MH, Park KH, Han WH, Yoon HJ, Jung HY, Hong JY, Chowdhury MR, Kim KY, Lee J, Song IS, Pang M, Choi MK, Gulbins E, Reichel M, Kornhuber J, Hong CW, Kim C, Kim SH, Schuchman EH, Jin HK, Bae JS. Choi BJ, et al. Nat Commun. 2023 Mar 24;14(1):1631. doi: 10.1038/s41467-023-37316-z. Nat Commun. 2023. PMID: 36959217 Free PMC article. - Passive immunotherapy for N-truncated tau ameliorates the cognitive deficits in two mouse Alzheimer's disease models.
Corsetti V, Borreca A, Latina V, Giacovazzo G, Pignataro A, Krashia P, Natale F, Cocco S, Rinaudo M, Malerba F, Florio R, Ciarapica R, Coccurello R, D'Amelio M, Ammassari-Teule M, Grassi C, Calissano P, Amadoro G. Corsetti V, et al. Brain Commun. 2020 Apr 6;2(1):fcaa039. doi: 10.1093/braincomms/fcaa039. eCollection 2020. Brain Commun. 2020. PMID: 32954296 Free PMC article. - A multi-targeting immunotherapy ameliorates multiple facets of Alzheimer's disease in 3xTg mice.
Feng X, Hou Y, Liu J, Yan F, Dai M, Chen M, Wang J, Li J, Liu Z, Sun D, Zhang Y, Yu X, Kong W, Wu H. Feng X, et al. NPJ Vaccines. 2024 Aug 20;9(1):153. doi: 10.1038/s41541-024-00942-9. NPJ Vaccines. 2024. PMID: 39164276 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG032611/AG/NIA NIH HHS/United States
- R01 AG020197/AG/NIA NIH HHS/United States
- R01 NS077239/NS/NINDS NIH HHS/United States
- R24 OD018339/OD/NIH HHS/United States
- R24 OD018340/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous