Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial - PubMed (original) (raw)
Clinical Trial
doi: 10.1016/S1470-2045(17)30900-2. Epub 2017 Dec 5.
John Ellerton 2, Jeffrey R Infante 3, Manish Agrawal 4, Michael Gordon 5, Raid Aljumaily 6, Carolyn D Britten 7, Luc Dirix 8, Keun-Wook Lee 9, Mathew Taylor 10, Patrick Schöffski 11, Ding Wang 12, Alain Ravaud 13, Arnold B Gelb 14, Junyuan Xiong 14, Galit Rosen 14, James L Gulley 15, Andrea B Apolo 16
Affiliations
- PMID: 29217288
- PMCID: PMC7984727
- DOI: 10.1016/S1470-2045(17)30900-2
Clinical Trial
Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial
Manish R Patel et al. Lancet Oncol. 2018 Jan.
Erratum in
- Correction to Lancet Oncol 2018; 19: 51-64.
[No authors listed] [No authors listed] Lancet Oncol. 2018 Jul;19(7):e335. doi: 10.1016/S1470-2045(18)30453-4. Epub 2018 Jun 29. Lancet Oncol. 2018. PMID: 30084379 No abstract available.
Abstract
Background: The approval of anti-programmed death ligand 1 (PD-L1) and anti-programmed death 1 agents has expanded treatment options for patients with locally advanced or metastatic urothelial carcinoma. Avelumab, a human monoclonal anti-PD-L1 antibody, has shown promising antitumour activity and safety in this disease. We aimed to assess the safety profile in patients (both post-platinum therapy and cisplatin-naive) treated with avelumab and to assess antitumour activity of this drug in post-platinum patients.
Methods: In this pooled analysis of two cohorts from the phase 1 dose-expansion JAVELIN Solid Tumor study, patients aged 18 years and older with histologically or cytologically confirmed locally advanced or metastatic urothelial carcinoma that had progressed after at least one previous platinum-based chemotherapy were enrolled from 80 cancer treatment centres or hospitals in the USA, Europe, and Asia. Eligible patients had adequate end-organ function, an Eastern Cooperative Oncology Group performance status of 0 or 1, life expectancy of at least 3 months, and at least one measurable lesion. Cisplatin-ineligible patients who might have been previously treated in the perioperative setting, including platinum-naive patients, were also eligible. Patients unselected for PD-L1 expression received avelumab (10 mg/kg, 1 h intravenous infusion) every 2 weeks until confirmed disease progression, unacceptable toxicity, or other criterion for withdrawal. The primary endpoint for this efficacy expansion cohort was confirmed best overall response (according to RECIST version 1.1), adjudicated by independent review. Safety analysis was done in all patients who received at least one dose of avelumab. Antitumour activity was assessed in post-platinum patients who received at least one dose of avelumab. This trial is registered with ClinicalTrials.gov, number NCT01772004; enrolment in this cohort of patients with metastatic urothelial carcinoma is closed and the trial is ongoing.
Findings: Between Sept 3, 2014, and March 15, 2016, 329 patients with advanced metastatic urothelial carcinoma were screened for enrolment into this study; 249 patients were eligible and received treatment with avelumab for a median of 12 weeks (IQR 6·0-19·7) and followed up for a median of 9·9 months (4·3-12·1). Safety and antitumour activity were evaluated at data cutoff on June 9, 2016. In 161 post-platinum patients with at least 6 months of follow-up, a best overall response of complete or partial response was recorded in 27 patients (17%; 95% CI 11-24), including nine (6%) complete responses and 18 (11%) partial responses. The most frequent treatment-related adverse events (any grade in ≥10% patients) were infusion-related reaction (73 [29%]; all grade 1-2) and fatigue (40 [16%]). Grade 3 or worse treatment-related adverse events occurred in 21 (8%) of 249 patients, the most common of which were fatigue (four [2%]), and asthenia, elevated lipase, hypophosphataemia, and pneumonitis in two (1%) patients each. 19 (8%) of 249 patients had a serious adverse event related to treatment with avelumab, and one treatment-related death occurred (pneumonitis).
Interpretation: Avelumab showed antitumour activity in the treatment of patients with platinum-refractory metastatic urothelial carcinoma; a manageable safety profile was reported in all avelumab-treated patients. These data provide the rationale for therapeutic use of avelumab in metastatic urothelial carcinoma and it has received accelerated US FDA approval in this setting on this basis.
Funding: Merck KGaA, and Pfizer Inc.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
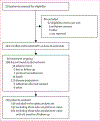
Figure 1:
Trial profile
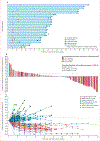
Figure 2:. Antitumour activity of avelumab
(A) Time and duration of confirmed responses in patients with at least 6 months of follow-up (27 confirmed responses as of data cutoff). (B) Change in size of target lesions from baseline in evaluable patients with at least 6 months of follow-up (n=130) with programmed death ligand 1 (PD-L1) expression status indicated (based on a ≥5% expression staining threshold on tumour cells; non-evaluable specimens [n=16] included those that were missing, of poor quality, or otherwise not available to provide results). The upper dotted line represents progression at 20% increase in size of target lesions and the lower dotted line represents the Response Evaluation Criteria In Solid Tumors (RECIST) boundary for complete response or partial response at 30% decrease in size of target lesions. (C) Percentage change in sum of target lesion diameters from baseline over time for all assessable patients with at least 6 months of follow-up (n=130), defined as those patients with baseline tumour assessments and at least one post-baseline assessment. The upper dotted line represents progression at 20% increase in size of target lesions and the lower dotted line represents the RECIST boundary for complete response or partial response at 30% decrease in size of target lesions.
Figure 3:. Subgroup analyses of responses based on patient and disease characteristics at baseline
(A) Objective responses by subgroup for select patient characteristics in patients with at least 6 months of follow-up. Plotted points represent % objective responses in each patient subgroup; error bars show 95% CIs. Vertical dashed line represents response rate in the n=161 population (17%). (B) Association of best overall response with tumour mutation based on RNAseq in patients with evaluable samples (n=29). ECOG=Eastern Cooperative Oncology Group. PD-L1=programmed death ligand 1.
Figure 4:. Progression-free survival (A) and overall survival (B) in patients with at least 6 months of follow-up (n=161) and according to PD-L1 expression status
PD-L1 expression based on a 5% expression staining threshold (n=139 evaluable). PD-L1=programmed death ligand 1.
Comment in
- JAVELIN: avelumab another spear to fight urothelial carcinoma.
Lalani AA, McGregor BA, Sonpavde GP, Choueiri TK. Lalani AA, et al. Lancet Oncol. 2018 Jan;19(1):5-7. doi: 10.1016/S1470-2045(17)30901-4. Epub 2017 Dec 5. Lancet Oncol. 2018. PMID: 29217289 No abstract available. - The role of avelumab in advanced urothelial carcinoma in the context of a dynamic treatment landscape.
Koshkin VS, Basu A, Grivas P. Koshkin VS, et al. Transl Cancer Res. 2019 Mar;8(Suppl 2):S130-S132. doi: 10.21037/tcr.2018.12.04. Transl Cancer Res. 2019. PMID: 35117082 Free PMC article. No abstract available.
Similar articles
- Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial.
Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, Heydebreck AV, Chin K, Cuillerot JM, Kelly K. Gulley JL, et al. Lancet Oncol. 2017 May;18(5):599-610. doi: 10.1016/S1470-2045(17)30240-1. Epub 2017 Mar 31. Lancet Oncol. 2017. PMID: 28373005 Free PMC article. Clinical Trial. - Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm, phase 2 trial.
Yu EY, Petrylak DP, O'Donnell PH, Lee JL, van der Heijden MS, Loriot Y, Stein MN, Necchi A, Kojima T, Harrison MR, Hoon Park S, Quinn DI, Heath EI, Rosenberg JE, Steinberg J, Liang SY, Trowbridge J, Campbell M, McGregor B, Balar AV. Yu EY, et al. Lancet Oncol. 2021 Jun;22(6):872-882. doi: 10.1016/S1470-2045(21)00094-2. Epub 2021 May 12. Lancet Oncol. 2021. PMID: 33991512 Clinical Trial. - Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial.
Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, Ogawa O, Park SH, Lee JL, De Giorgi U, Bögemann M, Bamias A, Eigl BJ, Gurney H, Mukherjee SD, Fradet Y, Skoneczna I, Tsiatas M, Novikov A, Suárez C, Fay AP, Duran I, Necchi A, Wildsmith S, He P, Angra N, Gupta AK, Levin W, Bellmunt J; DANUBE study investigators. Powles T, et al. Lancet Oncol. 2020 Dec;21(12):1574-1588. doi: 10.1016/S1470-2045(20)30541-6. Epub 2020 Sep 21. Lancet Oncol. 2020. PMID: 32971005 Clinical Trial. - Which place for avelumab in the management of urothelial carcinoma?
Larroquette M, Gross-Goupil M, Daste A, Robert G, Ravaud A, Domblides C. Larroquette M, et al. Expert Opin Biol Ther. 2019 Sep;19(9):863-870. doi: 10.1080/14712598.2019.1637412. Epub 2019 Jul 9. Expert Opin Biol Ther. 2019. PMID: 31286802 Review. - Avelumab as First-Line Maintenance Treatment in Locally Advanced or Metastatic Urothelial Carcinoma.
Mansinho A, Cruz A, Marconi L, Pinto C, Augusto I. Mansinho A, et al. Adv Ther. 2023 Oct;40(10):4134-4150. doi: 10.1007/s12325-023-02624-9. Epub 2023 Aug 22. Adv Ther. 2023. PMID: 37608243 Review.
Cited by
- The era of personalized treatments: Updates on immunotherapy within urothelial of bladder cancer.
Wu ZS, Wu S. Wu ZS, et al. Curr Urol. 2022 Sep;16(3):117-120. doi: 10.1097/CU9.0000000000000133. Epub 2022 Aug 27. Curr Urol. 2022. PMID: 36204361 Free PMC article. - Meta-analysis of immune-related adverse events of immune checkpoint inhibitor therapy in cancer patients.
Song P, Zhang D, Cui X, Zhang L. Song P, et al. Thorac Cancer. 2020 Sep;11(9):2406-2430. doi: 10.1111/1759-7714.13541. Epub 2020 Jul 8. Thorac Cancer. 2020. PMID: 32643323 Free PMC article. - A novel prognostic model based on cellular senescence-related gene signature for bladder cancer.
Luo L, Li F, Gong B, Xi P, Xie W. Luo L, et al. Front Oncol. 2022 Nov 23;12:937951. doi: 10.3389/fonc.2022.937951. eCollection 2022. Front Oncol. 2022. PMID: 36505846 Free PMC article. - PD-L2 amplification and durable disease stabilization in patient with urothelial carcinoma receiving pembrolizumab.
George S, Papanicolau-Sengos A, Lenzo FL, Conroy JM, Nesline M, Pabla S, Glenn ST, Burgher B, Andreas J, Giamo V, Qin M, Wang Y, Galluzzi L, Morrison C. George S, et al. Oncoimmunology. 2018 May 29;7(12):e1460298. doi: 10.1080/2162402X.2018.1460298. eCollection 2018. Oncoimmunology. 2018. PMID: 30524881 Free PMC article. - Immune landscape and immunotherapy for penile cancer.
Tang Y, Hu X, Wu K, Li X. Tang Y, et al. Front Immunol. 2022 Nov 29;13:1055235. doi: 10.3389/fimmu.2022.1055235. eCollection 2022. Front Immunol. 2022. PMID: 36524123 Free PMC article. Review.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortalitiy worldwide: IARC CancerBase no. 11. Lyon, France: International Agency for Research on Cancer. 2013. http://globocan.iarc.fr (accessed July 6, 2017).
- National Cancer Institute, Surveillance, Epidemiology, and End Results Program. SEER stat fact sheets: bladder cancer. 2016. https://seer.cancer.gov/statfacts/html/urinb.html (accessed July 6, 2017).
- Chism DD, Apolo AB, Milowsky MI. Chemotherapy for metastatic bladder cancer. In: Xylinas E, Shariat SF, eds. Advances in bladder cancer management. London, UK: Future Science Group, 2015: 206–20.
- Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 2010; 28: 1850–55. - PubMed
- NCCN. NCCN clinical practice guidelines in oncology (NCCN guidelines) for bladder cancer. 2017. https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed June 6, 2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials