Microglia: Driving critical periods and sexual differentiation of the brain - PubMed (original) (raw)
Review
. 2018 Jun;78(6):580-592.
doi: 10.1002/dneu.22569. Epub 2018 Jan 3.
Affiliations
- PMID: 29243403
- PMCID: PMC5980665
- DOI: 10.1002/dneu.22569
Review
Microglia: Driving critical periods and sexual differentiation of the brain
Jonathan W VanRyzin et al. Dev Neurobiol. 2018 Jun.
Abstract
The proverbial role of microglia during brain development is shifting from passive members of the brain's immune system to active participants that are able to dictate enduring outcomes. Despite these advances, little attention has been paid to one of the most critical components of early brain development-sexual differentiation. Mounting evidence suggests that the normal developmental functions microglia perform-cell number regulation and synaptic connectivity-may be involved in the sex-specific patterning of the brain during these early sensitive periods, and may have lasting sex-dependent and sex-independent effects on behavior. In this review, we outline the known functions of microglia during developmental sensitive periods, and highlight the role they play in the establishment of sex differences in brain and behavior. We also propose a framework for how researchers can incorporate microglia in their study of sex differences and vice versa. © 2017 Wiley Periodicals, Inc. Develop Neurobiol 78: 580-592, 2018.
Keywords: behavior; brain development; critical (sensitive) period; microglia; neuroimmunology; sex differences; sexual differentiation.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest statement: No conflicts of interest
Figures
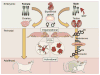
Figure 1. Sexual differentiation of the brain and behavior
A male fetus carries a gene on the SRY gene on the Y chromosome and this gene codes for a protein called Testis Determining Factor which differentiates the biopotential gonad into testes. The testes synthesize testosterone which is converted to estradiol in some areas of the brain by the enzyme aromatase. In the preoptic area, estradiol exposure in males and females ultimately triggers microglia to assume an ameboid morphology and begin producing prostaglandin (PGE2) which acts on neurons to organize a 2-fold higher synaptic density pattern that is necessary for male copulatory behavior later in life. Post-puberty and in adulthood the organized (masculinized) POA is activated by circulating gonadal steroids to enable male sexual behavior.
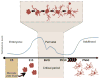
Figure 2. Microglial morphology is variable during the critical period for sexual differentiation
Microglia originate in the yolk sac and migrate into the brain during embryogenesis. These cells are capable of morphological plasticity at all stages of life during and after insult. Under normal conditions, however, microglia exhibit an especially dynamic array of morphologies during the critical period for sexual differentiation of the brain. These morphologies range from (left to right above) ameboid, stout, transitioning, ramified and phagocytic. The critical period for sexual differentiation of the brain is defined by the surge in testicular hormones and their metabolites in males perinatally. Sex differences in microglial morphologies during the critical period have been identified in many regions of the brain, however, these differences often disappear, or are even reversed, at the close of the critical period for sexual differentiation.
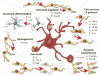
Figure 3. Microglia modulate sexually differentiated endpoints
During the critical period for sexual differentiation microglia help to modulate fundamental processes from cell genesis to migration, differentiation, synaptogenesis and cell death, all of which differ in males and females in at least one brain region. Abbreviations: CR3, complement receptor 3; DAP12, DNAX-activation protein 12; Insulin-like growth factor 1; IL, interleukin; LIF, leukemia inhibitory factor; NF-kB, nuclear factor-kappaB; NGF, nerve growth factor; Tim-4, Tcell, immunoglobulin, mucin 4.
Similar articles
- Sexual differentiation of microglia.
Villa A, Della Torre S, Maggi A. Villa A, et al. Front Neuroendocrinol. 2019 Jan;52:156-164. doi: 10.1016/j.yfrne.2018.11.003. Epub 2018 Nov 24. Front Neuroendocrinol. 2019. PMID: 30481522 Review. - A starring role for microglia in brain sex differences.
Lenz KM, McCarthy MM. Lenz KM, et al. Neuroscientist. 2015 Jun;21(3):306-21. doi: 10.1177/1073858414536468. Epub 2014 May 28. Neuroscientist. 2015. PMID: 24871624 Free PMC article. Review. - Microglia and sexual differentiation of the developing brain: A focus on ontogeny and intrinsic factors.
Bordt EA, Ceasrine AM, Bilbo SD. Bordt EA, et al. Glia. 2020 Jun;68(6):1085-1099. doi: 10.1002/glia.23753. Epub 2019 Nov 19. Glia. 2020. PMID: 31743527 Free PMC article. Review. - Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function.
Lenz KM, Nelson LH. Lenz KM, et al. Front Immunol. 2018 Apr 13;9:698. doi: 10.3389/fimmu.2018.00698. eCollection 2018. Front Immunol. 2018. PMID: 29706957 Free PMC article. Review. - Sexual dimorphism of microglia and synapses during mouse postnatal development.
Weinhard L, Neniskyte U, Vadisiute A, di Bartolomei G, Aygün N, Riviere L, Zonfrillo F, Dymecki S, Gross C. Weinhard L, et al. Dev Neurobiol. 2018 Jun;78(6):618-626. doi: 10.1002/dneu.22568. Epub 2018 Jan 4. Dev Neurobiol. 2018. PMID: 29239126 Free PMC article.
Cited by
- Microglial process convergence onto injured axonal swellings, a human postmortem brain tissue study.
Logan-Wesley AL, Gorse KM, Lafrenaye AD. Logan-Wesley AL, et al. Sci Rep. 2024 Sep 12;14(1):21369. doi: 10.1038/s41598-024-71312-7. Sci Rep. 2024. PMID: 39266604 Free PMC article. - Feminization of social play behavior depends on microglia.
VanRyzin JW, Marquardt AE, McCarthy MM. VanRyzin JW, et al. bioRxiv [Preprint]. 2024 Aug 19:2024.08.19.608675. doi: 10.1101/2024.08.19.608675. bioRxiv. 2024. PMID: 39229086 Free PMC article. Preprint. - Viral-mediated inflammation by Poly I:C induces the chemokine CCL5 in NK cells and its receptors CCR1 and CCR5 in microglia in the neonatal rat cerebellum.
Perez-Pouchoulen M, Holley AS, Reinl EL, VanRyzin JW, Mehrabani A, Dionisos C, Mirza M, McCarthy MM. Perez-Pouchoulen M, et al. NeuroImmune Pharm Ther. 2024 Apr 23;3(2):155-168. doi: 10.1515/nipt-2024-0002. eCollection 2024 Jun. NeuroImmune Pharm Ther. 2024. PMID: 39175524 Free PMC article. - The arrangements of the microvasculature and surrounding glial cells are linked to blood-brain barrier formation in the cerebral cortex.
Shigemoto-Mogami Y, Nakayama-Kitamura K, Sato K. Shigemoto-Mogami Y, et al. Front Neuroanat. 2024 Aug 7;18:1438190. doi: 10.3389/fnana.2024.1438190. eCollection 2024. Front Neuroanat. 2024. PMID: 39170850 Free PMC article. - Microglial process convergence onto injured axonal swellings, a human postmortem brain tissue study.
Logan-Wesley AL, Gorse KM, Lafrenaye AD. Logan-Wesley AL, et al. Res Sq [Preprint]. 2024 Aug 9:rs.3.rs-4713316. doi: 10.21203/rs.3.rs-4713316/v1. Res Sq. 2024. PMID: 39149456 Free PMC article. Updated. Preprint.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. - PubMed
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. - PubMed
- Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. - PubMed
- Antony JM, Paquin A, Nutt SL, Kaplan DR, Miller FD. Endogenous microglia regulate development of embryonic cortical precursor cells. J Neurosci Res. 2011;89:286–298. - PubMed
Publication types
MeSH terms
Grants and funding
- F31 NS093947/NS/NINDS NIH HHS/United States
- R01 DA039062/DA/NIDA NIH HHS/United States
- R01 MH052716/MH/NIMH NIH HHS/United States
- R01 MH091424/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources